Question: Thanks, not really sure how to do this. Determine the standard enthalpy change for each of the following reactions. Report your answers to the nearest
Thanks, not really sure how to do this. 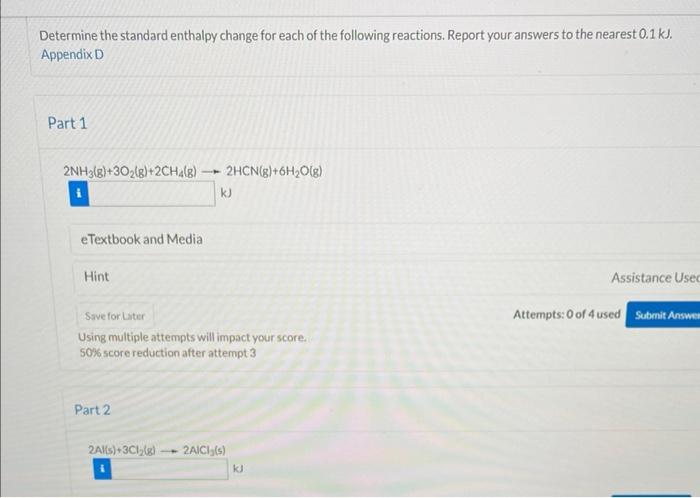
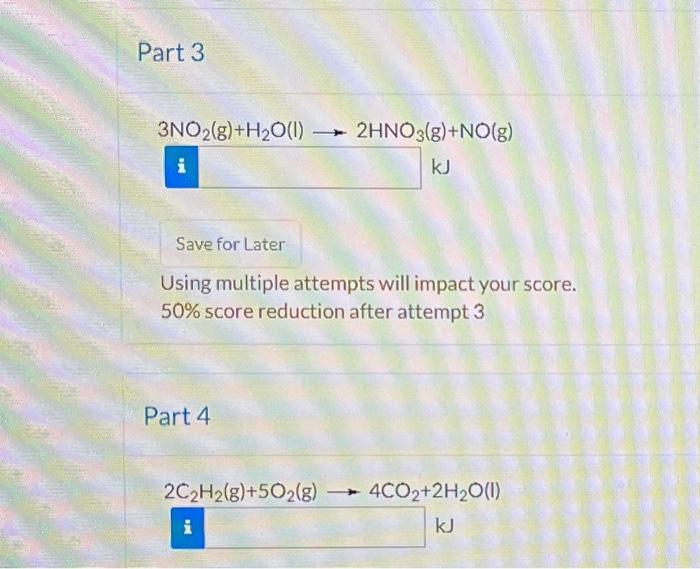


Determine the standard enthalpy change for each of the following reactions. Report your answers to the nearest 0.1kJ. Appendix D Part 1 2NH3(g)+3O2(g)+2CH4(g)2HCN(g)+6H2O(g) kJ eTextbook and Media Hint Attempts: 0 of 4 used Using multiple attempts will impact your score. 50% score reduction after attempt 3 Part 2 2A(s)+3C2(s)2ACCI(s) 3NO2(g)+H2O(I)2HNO3(g)+NO(g) Using multiple attempts will impact your score. 50% score reduction after attempt 3 Part 4 2C2H2(g)+5O2(g)4CO2+2H2O(I)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


