Question: That's the question and also the hints for doing Q4: Gas Phase Reaction Q4. Ethene reacts with hydrogen in the presence of a finely divided
That's the question and also the hints for doing
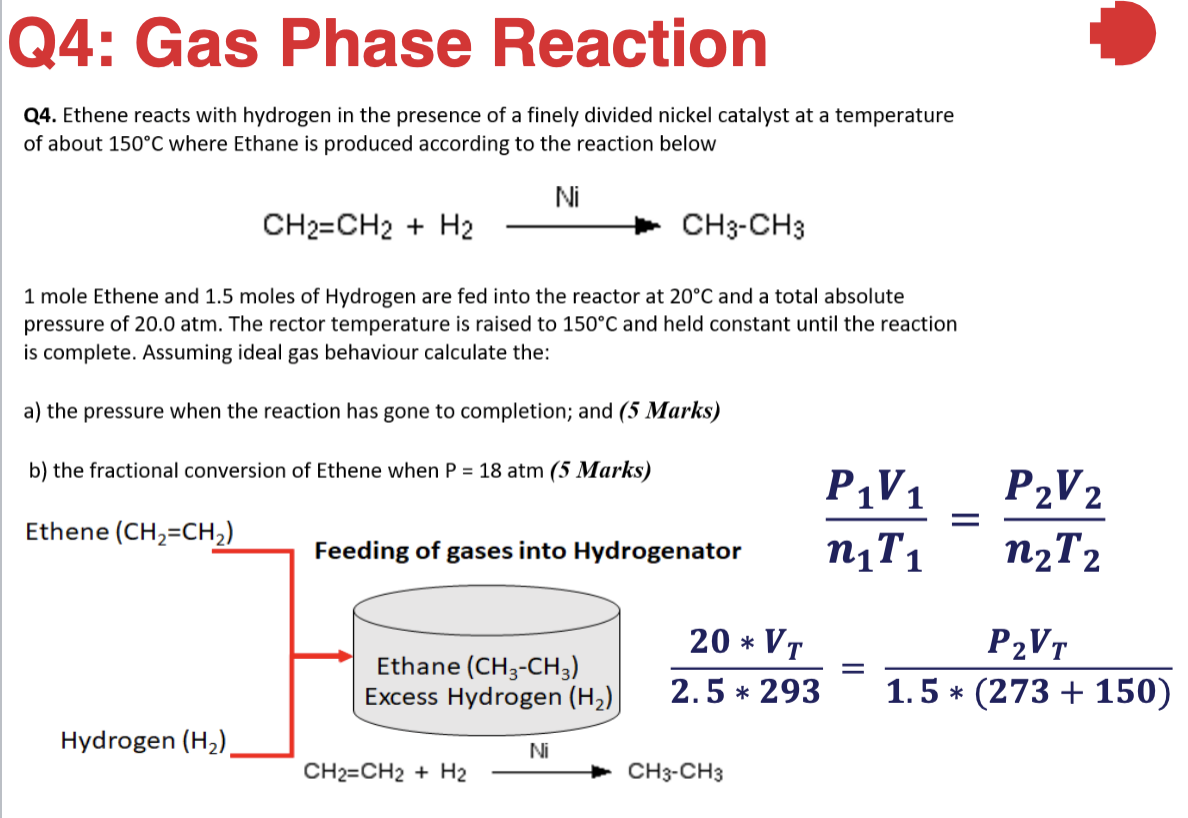
Q4: Gas Phase Reaction Q4. Ethene reacts with hydrogen in the presence of a finely divided nickel catalyst at a temperature of about 150C where Ethane is produced according to the reaction below Ni CH2=CH2 + H2 CH3-CH3 1 mole Ethene and 1.5 moles of Hydrogen are fed into the reactor at 20C and a total absolute pressure of 20.0 atm. The rector temperature is raised to 150C and held constant until the reaction is complete. Assuming ideal gas behaviour calculate the: a) the pressure when the reaction has gone to completion; and (5 Marks) b) the fractional conversion of Ethene when P = 18 atm (5 Marks) P1V1 niT1 = Ethene (CH2=CH2) P2V2 n2T2 Feeding of gases into Hydrogenator 20 * V. Ethane (CH3-CH3) Excess Hydrogen (H2) P2VT 1.5 * (273 + 150) 2.5 * 293 Hydrogen (H2) Ni CH2=CH2 + H2 CH3-CH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


