Question: That's the question and also the hints for doing Transfer of CO2 from pressurized tank to gas holder 8.314 m. Pa/(molK) P3,V3, T3, n3 1
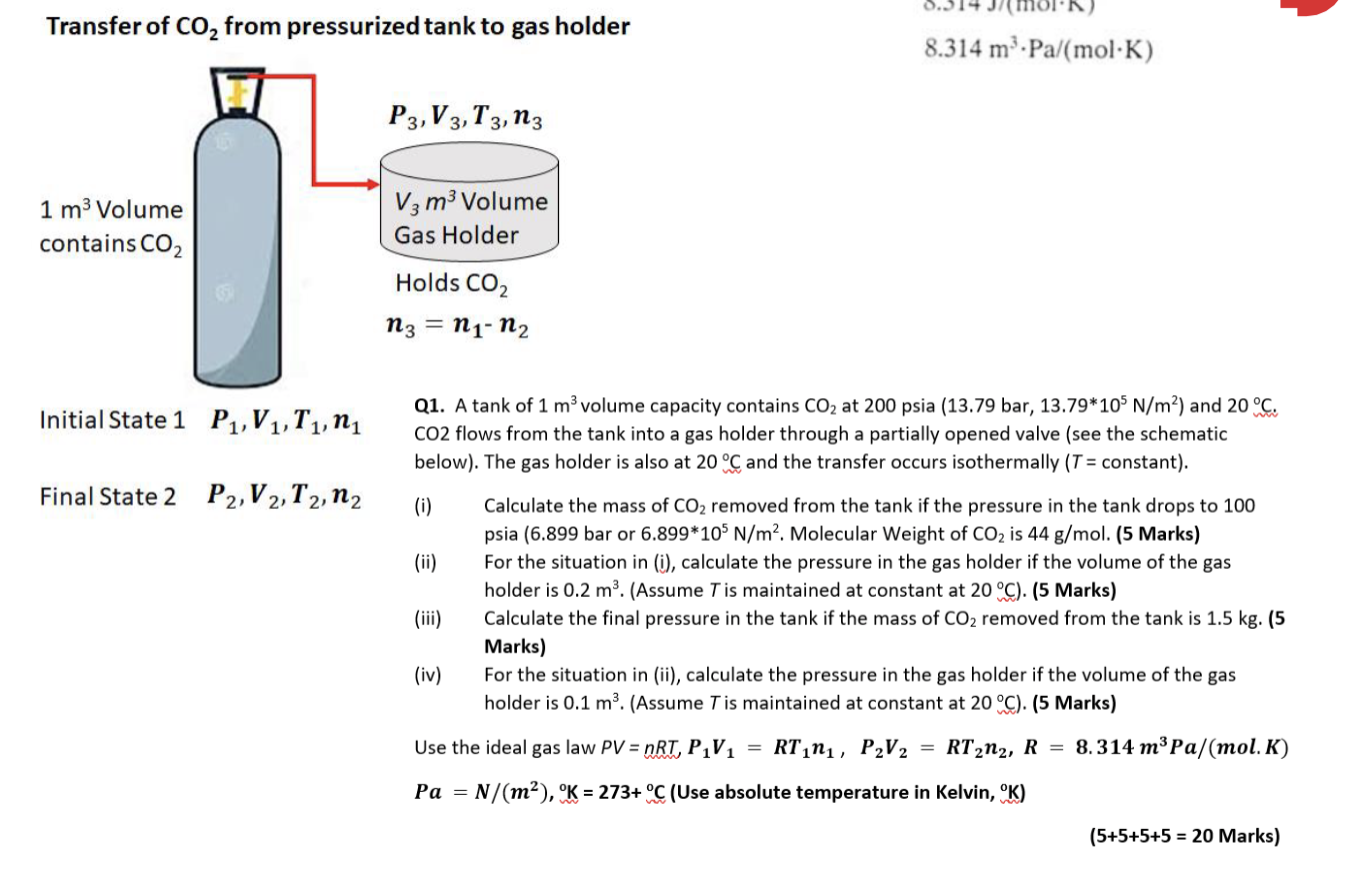
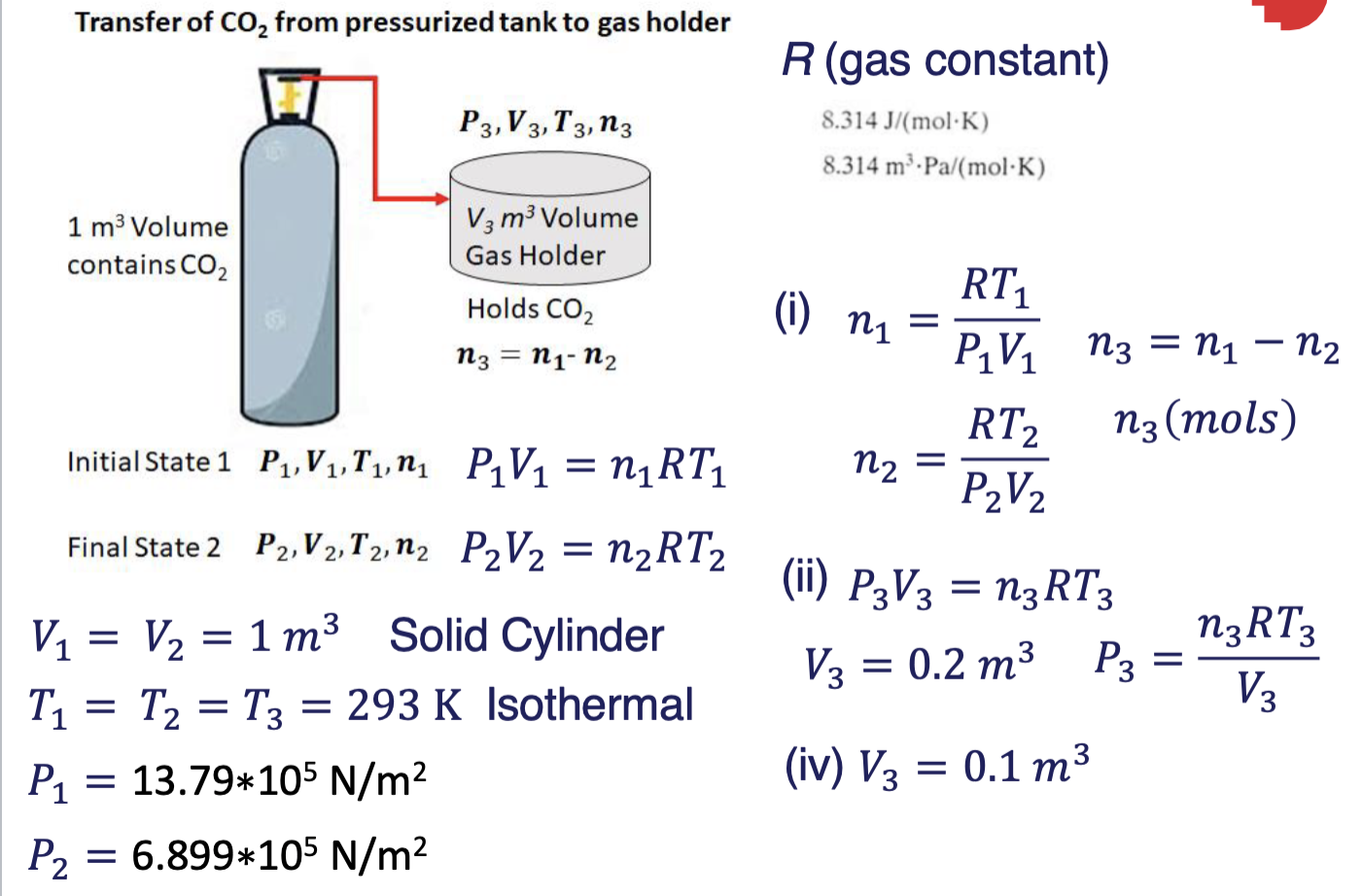
That's the question and also the hints for doing
Transfer of CO2 from pressurized tank to gas holder 8.314 m. Pa/(molK) P3,V3, T3, n3 1 m3 Volume contains CO2 V3 m Volume Gas Holder Holds CO2 n3 = n1-n2 Initial State 1 P1,V1,T1,ny Q1. A tank of 1 m3 volume capacity contains CO2 at 200 psia (13.79 bar, 13.79*105 N/m?) and 20 C. CO2 flows from the tank into a gas holder through a partially opened valve (see the schematic below). The gas holder is also at 20 C and the transfer occurs isothermally (T = constant). Final State 2 P2,V2, T2, n2 (0) Calculate the mass of CO2 removed from the tank if the pressure in the tank drops to 100 psia (6.899 bar or 6.899*105 N/m2. Molecular Weight of CO2 is 44 g/mol. (5 Marks) (ii) For the situation in (i), calculate the pressure in the gas holder if the volume of the gas holder is 0.2 m3. (Assume T is maintained at constant at 20C). (5 Marks) (iii) Calculate the final pressure in the tank if the mass of CO2 removed from the tank is 1.5 kg. (5 Marks) (iv) For the situation in (ii), calculate the pressure in the gas holder if the volume of the gas holder is 0.1 m2. (Assume T is maintained at constant at 20C). (5 Marks) Use the ideal gas law PV = nRT, P1V1 = RTini, P2V2 = RT212, R = 8.314 mPa/mol. K) Pa = N/(m), K = 273+ C (Use absolute temperature in Kelvin, CK) (5+5+5+5 = 20 Marks) Transfer of Co, from pressurized tank to gas holder R (gas constant) P3,V3,T3, n3 8.314 J/(mol.K) 8.314 m.Pa/(molK) 1 m3 Volume contains CO2 V3 m3 Volume Gas Holder Holds CO2 (i) ni = n3 = n1-n2 = RT1 P1V1 n3 = nu n2 n3(mols) P2V2 RT2 Initial State 1 P1V1,T, PV = n(RT1 1 = n2 = Final State 2 P2, V2, T2,12 P2 V2 = n2RT2 = = (ii) P3V3 = n3RT3 P3 = = V3 = 0.2 m3 n3RT3 V3 = = = = Vi = V2 = 1 m3 Solid Cylinder T1 = T2 = T3 = 293 K Isothermal P1 = 13.79*105 N/m2 : 6.899*105 N/m2 (iv) V3 = 0.1 m3 P2 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


