Question: The 2s atomic orbital for hydrogen-like atom is given as: 3/2 428 = 1 32 (21) ao 2 Zr ao -Zr/2a0 e How many
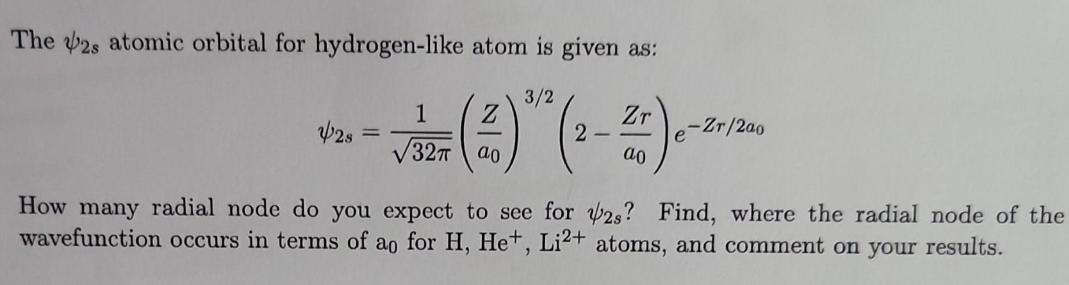
The 2s atomic orbital for hydrogen-like atom is given as: 3/2 428 = 1 32 (21) ao 2 Zr ao -Zr/2a0 e How many radial node do you expect to see for 2s? Find, where the radial node of the wavefunction occurs in terms of ao for H, He+, Li2+ atoms, and comment on your results.
Step by Step Solution
There are 3 Steps involved in it
To find the number of radial nodes for the wavefunction 2s we need to determine the number of points ... View full answer

Get step-by-step solutions from verified subject matter experts


