Question: THE 3 SELECTED CHOICES IS NOT THE CORRECT ANSWER! Select all of the correct statements about reaction quotients and equilibrium constants from the choices below.
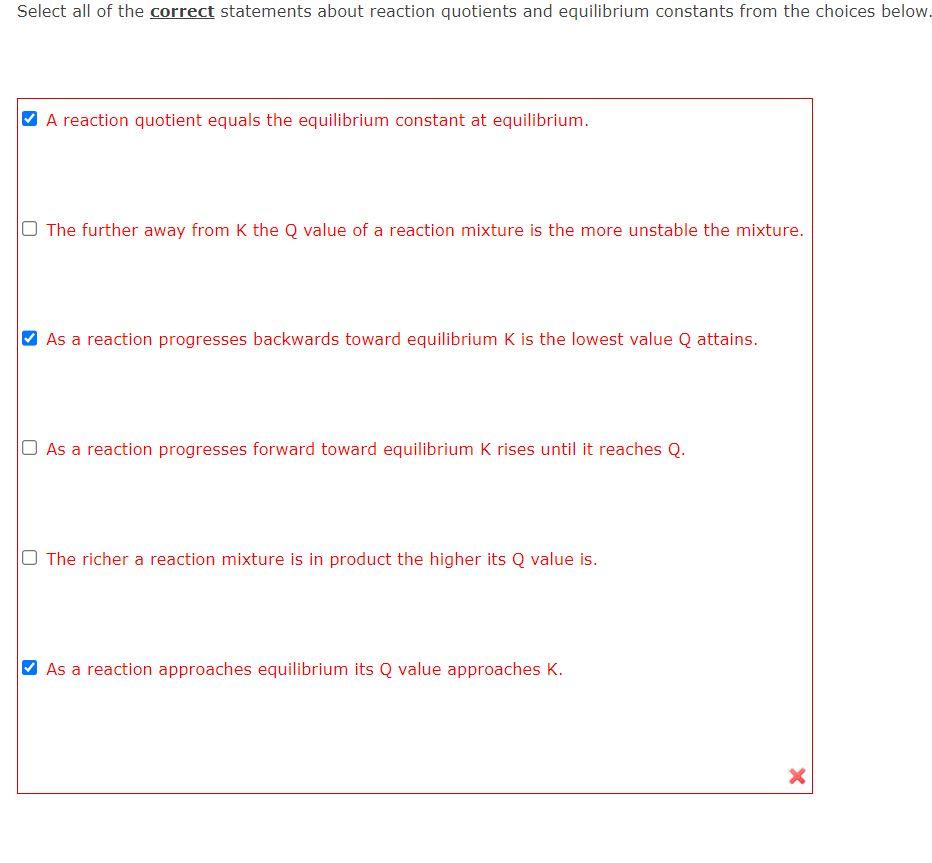
THE 3 SELECTED CHOICES IS NOT THE CORRECT ANSWER!
Select all of the correct statements about reaction quotients and equilibrium constants from the choices below. A reaction quotient equals the equilibrium constant at equilibrium. The further away from K the Q value of a reaction mixture is the more unstable the mixture. As a reaction progresses backwards toward equilibrium K is the lowest value Q attains. As a reaction progresses forward toward equilibrium K rises until it reaches Q. The richer a reaction mixture is in product the higher its Q value is. As a reaction approaches equilibrium its Q value approaches K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


