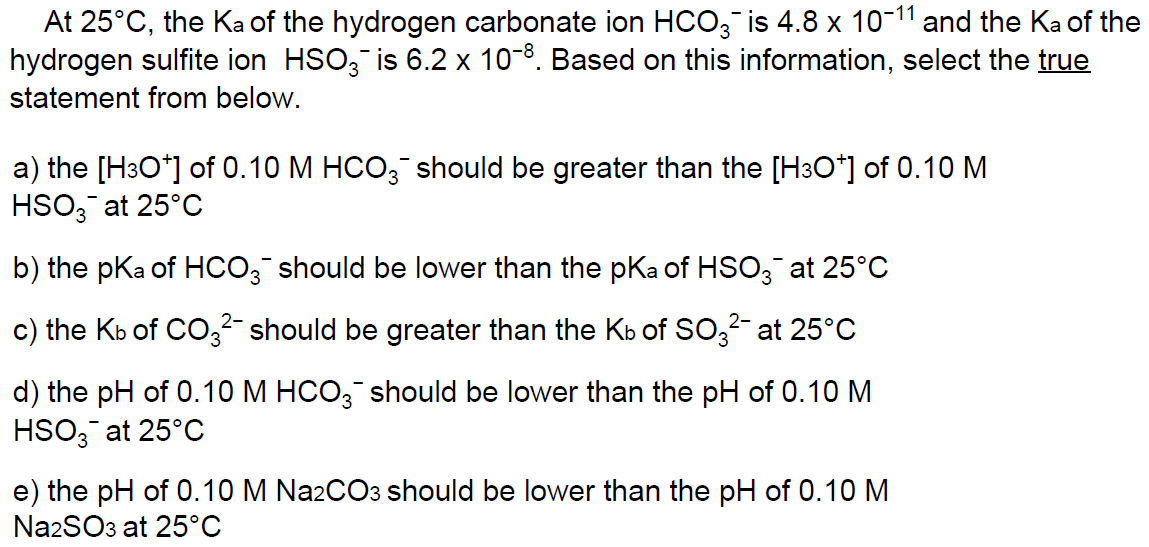
Question: THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION. At 25C, the Ka of the hydrogen carbonate ion HCO3 is 4.8 x 10-1 and the
 THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION.
THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION.
At 25C, the Ka of the hydrogen carbonate ion HCO3 is 4.8 x 10-1 and the Ka of the hydrogen sulfite ion HSO3 is 6.2 x 10-8. Based on this information, select the true statement from below. a) the [H3O+] of 0.10 M HCO3 should be greater than the [H3O+] of 0.10 M HSO3 at 25C b) the pKa of HCO3 should be lower than the pKa of HSO3 at 25C c) the Kb of CO3- should be greater than the Kb of SO3- at 25C d) the pH of 0.10 M HCO3 should be lower than the pH of 0.10 M HSO3 at 25C e) the pH of 0.10 M Na2CO3 should be lower than the pH of 0.10 M Na2SO3 at 25C
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Answer Given Ka H 0 40X1011 19 48X1011 11 065 1035 2 true statement Answer ... View full answer

Get step-by-step solutions from verified subject matter experts


