Question: the answer is not +/- 0.02. MISSED THIS? Read Section 15 3 (Engos 837642) Watch KCV 153 The graph below shows a plot of the
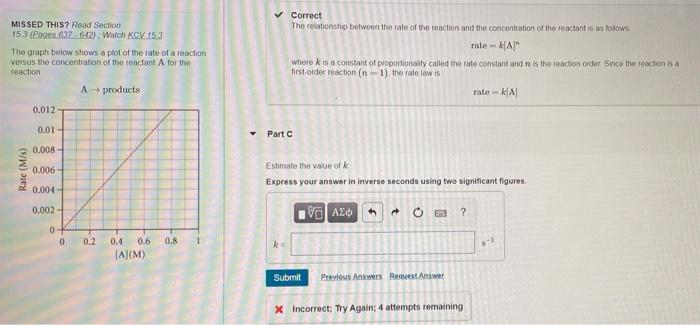
MISSED THIS? Read Section 15 3 (Engos 837642) Watch KCV 153 The graph below shows a plot of the rate of a reaction versus the concentration of the reactant A for the reaction A products Correct The relationship between the rate of the roaction and the concentration of the reactant is as follows rate -AA" where is a constant of proportionally called the rate constant and is the reaction order since the reaction is a first-order reaction (n-1), the rate law is rate KA 0.012 0.01 Part 0.008 Rate (M/s) 0.006 Estimate the value of Express your answer in Inverse seconds using two significant figures. 0.004- 0,002- V AED to + 0 19 ? 0 0 0.2 0.8 ! 0.4 0.6 TA(M) Submit Previous Answers Request Answer X Incorrect: Try Again: 4 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


