Question: the answer is supposedly 1589 torr but i keep getting a different answer can you help ? 8) Consider the apparatus pictured below, which consists
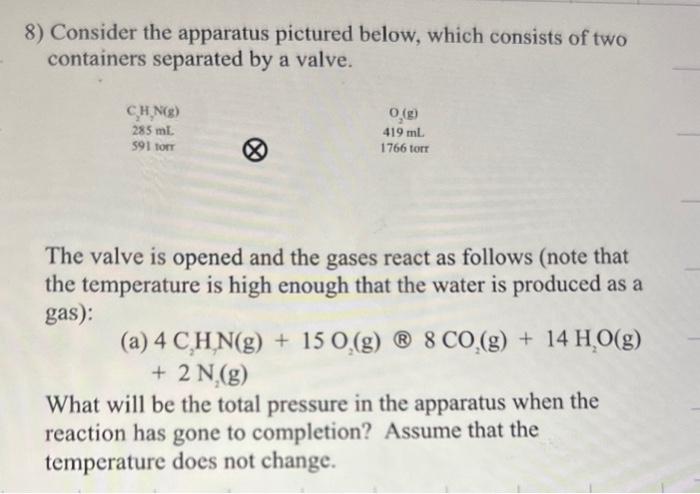
8) Consider the apparatus pictured below, which consists of two containers separated by a valve. The valve is opened and the gases react as follows (note that the temperature is high enough that the water is produced as a gas): (a)+4C2H1N(g)+15O2(g)(B)8CO2(g)+14H2O(g)2N2(g) What will be the total pressure in the apparatus when the reaction has gone to completion? Assume that the temperature does not change
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


