Question: The answer they posted is wrong. Please do not copy other work directly! Wish you have a good day. 3. Consider a fluid obeying the
The answer they posted is wrong. Please do not copy other work directly! Wish you have a good day.
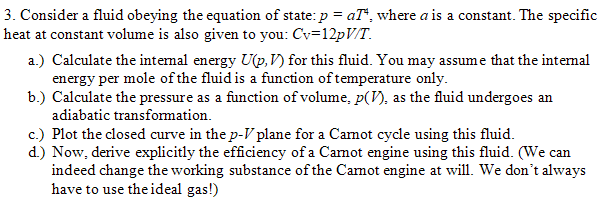
3. Consider a fluid obeying the equation of state: p=aT4, where a is a constant. The specific heat at constant volume is also given to you: CV=12pV/T. a.) Calculate the internal energy U(p,V) for this fluid. You may assume that the internal energy per mole of the fluid is a function of temperature only. b.) Calculate the pressure as a function of volume, p(V), as the fluid undergoes an adiabatic transformation. c.) Plot the closed curve in the pV plane for a Camot cycle using this fluid. d.) Now, derive explicitly the efficiency of a Camot engine using this fluid. (We can indeed change the working substance of the Camot engine at will. We don't always have to use the ideal gas!)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


