Question: the appendix f is linked below! Using the additivity constants found in appendix F of your lab manual, calculate the approximate chemical shifts of the

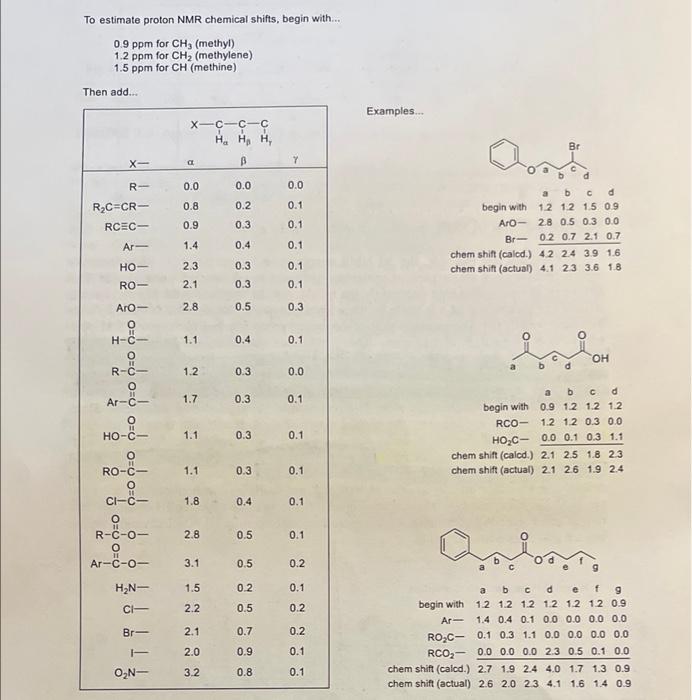
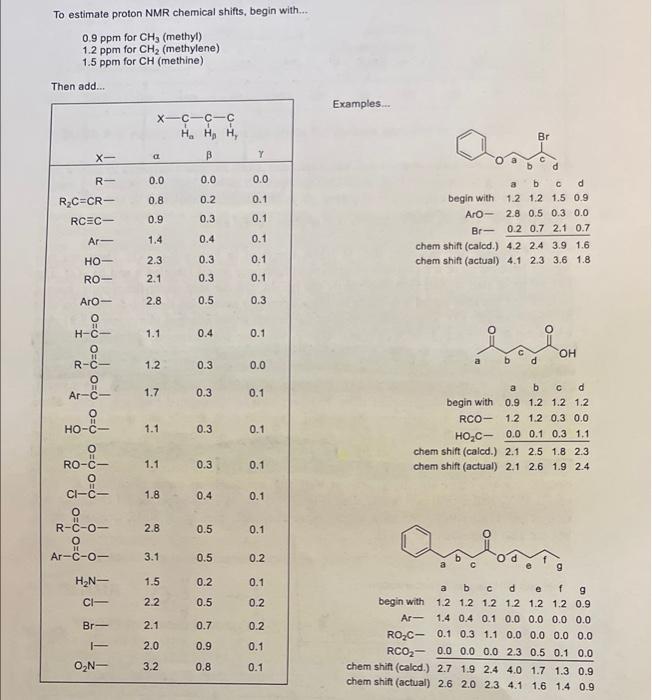
Using the additivity constants found in appendix F of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!l!, 2.5 points) To estimate proton NMR chemical shifts, begin with... 0.9ppm for CH3 (methyl) 1.2ppm for CH2 (methylene) 1.5ppm for CH (methine) Then add... Examples... To estimate proton NMR chemical shifts, begin with... 0.9ppm for CH3 (methyl) 1.2 ppm for CH2 (methylene) 1.5ppm for CH (methine) Than add.. Examples... \begin{tabular}{rcccc} & a & b & c & d \\ begin with & 1.2 & 1.2 & 1.5 & 0.9 \\ ArO - & 2.8 & 0.5 & 0.3 & 0.0 \\ Br- & 0.2 & 0.7 & 2.1 & 0.7 \\ \cline { 2 - 5 } chem shift (calod.) & 4.2 & 2.4 & 3.9 & 1.6 \\ chem shift (actual) & 4.1 & 2.3 & 3.6 & 1.8 \end{tabular} chemshift(actual)2.12.61.92.4 beginwithArRO2CRCO2shift(caled.)shift(actual)a1.21.40.10.02.72.6b1.20.40.30.01.92.0c1.20.11.10.02.42.3d1.20.00.02.34.04.1e1.20.00.00.51.71.6f1.20.00.00.11.31.4g0.90.00.00.00.90.9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


