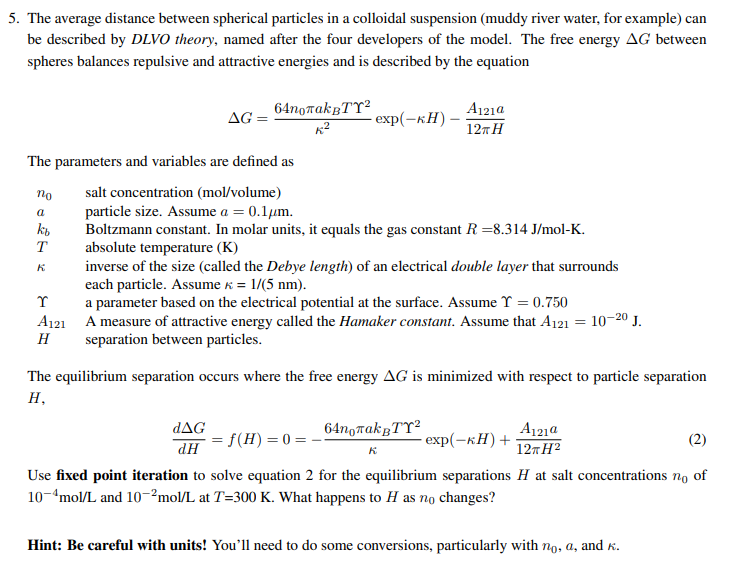
Question: The average distance between spherical particles in a colloidal suspension ( muddy river water, for example ) can be described by DLVO theory, named after
The average distance between spherical particles in a colloidal suspension muddy river water, for example can
be described by DLVO theory, named after the four developers of the model. The free energy between
spheres balances repulsive and attractive energies and is described by the equation
exp
The parameters and variables are defined as
salt concentration olume
a particle size. Assume
Boltzmann constant. molar units, equals the gas constant
temperature
inverse the size the Debye length electrical double layer that surrounds
each particle. Assume
a parameter based the electrical potential the surface. Assume
separation between particles.
The equilibrium separation occurs where the free energy is minimized with respect to particle separation
H
exp
Use fixed point iteration to solve equation for the equilibrium separations at salt concentrations of
and at What happens to as changes?
Hint: Be careful with units! You'll need to do some conversions, particularly with and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


