Question: The binary mixture methanol (1) benzene (2) forms an azetrope at a pressure P = 1.013 bar (= 1 atm) and T = 331.10 K.
The binary mixture methanol (1) benzene (2) forms an azetrope at a pressure P = 1.013 bar (= 1 atm) and T = 331.10 K. The composition of the azetrope is x1 = 0.614.
Critical data:
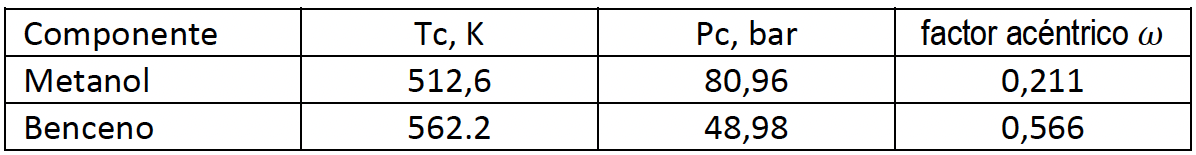 PART A Calculate the vapor fugacities for components 1 and 2, using the following models: That is, calculate 1, 2. Check your calculations, it's easy to make a mistake.
PART A Calculate the vapor fugacities for components 1 and 2, using the following models: That is, calculate 1, 2. Check your calculations, it's easy to make a mistake.
Model 1 - ideal gas solution, yi=1
Apply the Lewis and Randall Rule, which says: fi=yifi fi is the fugacity of the pure component, which can be calculated as follows (you can choose): a) With the virial equation of two terms:
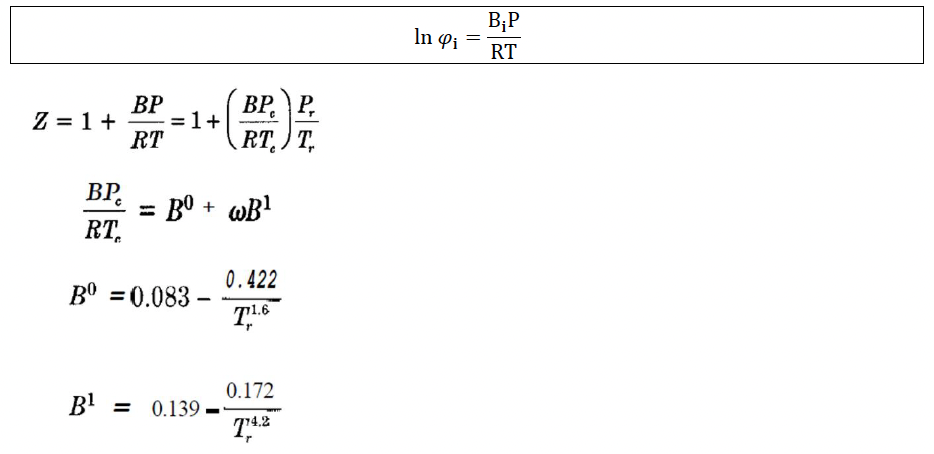
With a generalized correlation (Tables E15 and E16 book Smith, Van Ness & Abbott):
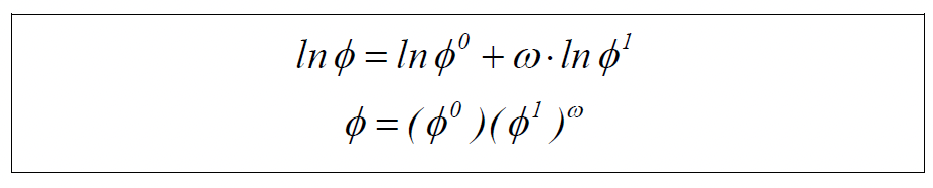
Model 2: Real Gas Solution, Virial Equation
See applicable equations and example 11.9 on pages 409 to 411 of the book by Smith, Van Ness & Abbott.
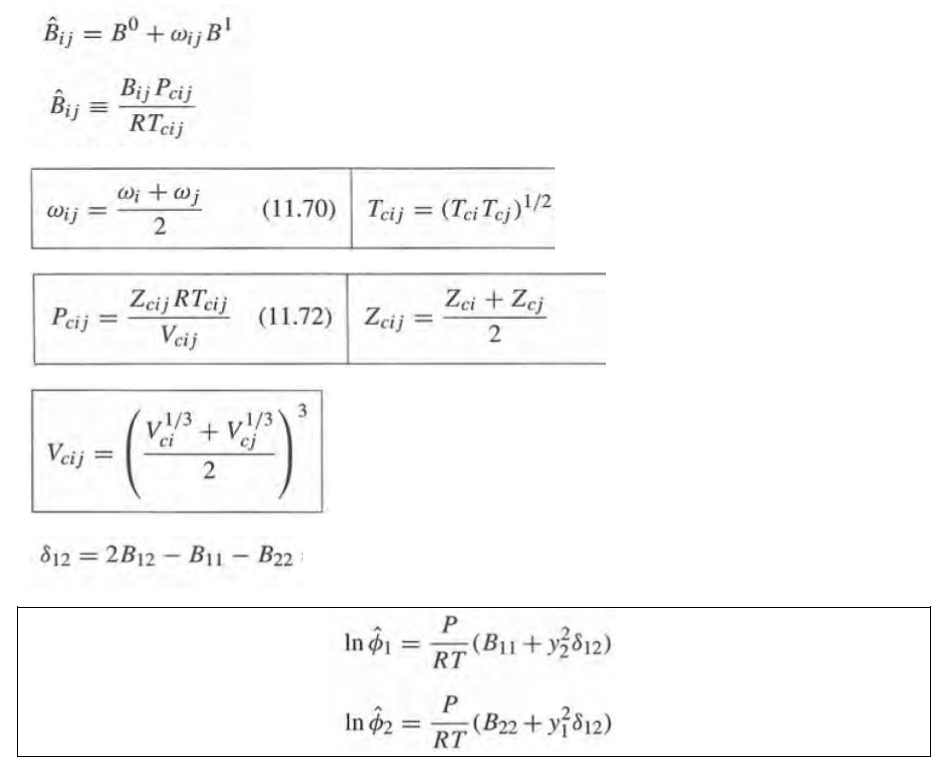
Pc, bar Componente Metanol Benceno Tc, K 512,6 562.2 80,96 48,98 factor acntrico w 0,211 0,566 In Qi B;P RT BP BP. P, Z=1+ = 1+ RT RT,)T, CE BP. RT. = BO + wB1 B =0.083 - 0.422 T1.6 ! Bl = = 0.172 0.139 - 7.4.2 = (19)(,0)=0 : 0 In $ = In '+oIn ' Bij = B + Wi;B! Bij Pcij Bij = RTcij Wij Wi+w; 2 (11.70) Tcij = (Tci Tcj)1/2 cij Zcij RTcij Veij (11.72) Zcij Zcit Zej 2 3 v!'3+ v. 1/3 Vcij II 2 812 = 2B12 - B1 - B22 - In 1 (Bu1 + y2812) RT In 02 (B22 + y 812) RT Pc, bar Componente Metanol Benceno Tc, K 512,6 562.2 80,96 48,98 factor acntrico w 0,211 0,566 In Qi B;P RT BP BP. P, Z=1+ = 1+ RT RT,)T, CE BP. RT. = BO + wB1 B =0.083 - 0.422 T1.6 ! Bl = = 0.172 0.139 - 7.4.2 = (19)(,0)=0 : 0 In $ = In '+oIn ' Bij = B + Wi;B! Bij Pcij Bij = RTcij Wij Wi+w; 2 (11.70) Tcij = (Tci Tcj)1/2 cij Zcij RTcij Veij (11.72) Zcij Zcit Zej 2 3 v!'3+ v. 1/3 Vcij II 2 812 = 2B12 - B1 - B22 - In 1 (Bu1 + y2812) RT In 02 (B22 + y 812) RT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


