Question: The binding constants for Mg2+ and Ca2+ are too similar for the species to be determined independently by titration without proper treatment. When a dilute
The binding constants for Mg2+ and Ca2+ are too similar for the species to be determined independently by titration without proper treatment. When a dilute NaOH solution is added to the tap water (instead of a buffer), the species can be separated because of the formation of a precipitate. Examine Appendix 2 from Fundamentals of Analytical Chemistry. What is the precipitate that is formed? What species remains in solution and reacts with the EDTA during the titration? What is the name (vocab question) for the role the hydroxide ion (OH-) plays in the process?
appendix 2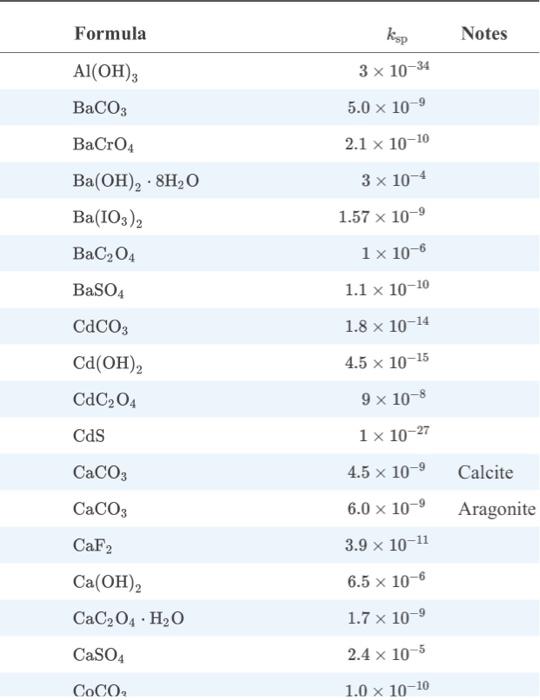
Formula Al(OH)3 BaCO3 BaCrO4 Ba(OH) 8HO Ba(103) 2 BaC04 BaSO4 CdCO3 Cd (OH) 2 CdC04 Cds CaCO3 CaCO3 CaF2 . Ca(OH) CaCO4 HO CaSO4 COCO ksp 3 x 10-34 5.0 10- 2.1 x 10-10 3 x 10-4 1.57 10- 1 x 10-6 1.1 x 10-10 1.8 x 10-14 4.5 x 10-15 9 x 10-8 1 x 10-27 4.5 x 10- 6.0 10- 3.9 10-11 6.5 x 10-6 1.7 x 10- 2.4 x 10-5 1.0 10-10 Notes Calcite Aragonite
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


