Question: The conversion to bars is confusing me The following reaction was carried out in a 2.25 L reaction vessel at 1100 K: C(s) +H2O(g) =
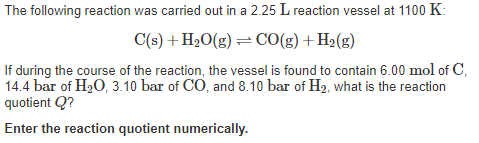
The conversion to bars is confusing me
The following reaction was carried out in a 2.25 L reaction vessel at 1100 K: C(s) +H2O(g) = CO(g) + H2(g) If during the course of the reaction, the vessel is found to contain 6.00 mol of C, 14.4 bar of H20, 3.10 bar of CO, and 8.10 bar of H2, what is the reaction quotient Q? Enter the reaction quotient numerically
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


