Question: THE CORRECT ANSWER IS 2.39 not 0.72!!! A liquid phase reaction, AB+C, is to be carried out in an isothermal, well mixed batch reactor with
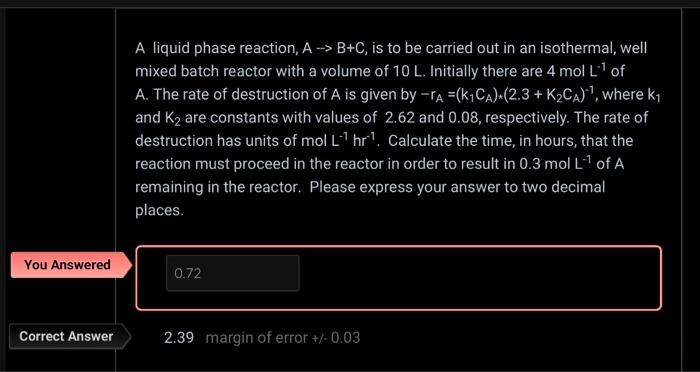
A liquid phase reaction, AB+C, is to be carried out in an isothermal, well mixed batch reactor with a volume of 10L. Initially there are 4molL1 of A. The rate of destruction of A is given by rA=(k1CA)(2.3+K2CA)1, where k1 and K2 are constants with values of 2.62 and 0.08, respectively. The rate of destruction has units of molL1hr1. Calculate the time, in hours, that the reaction must proceed in the reactor in order to result in 0.3molL1 of A remaining in the reactor. Please express your answer to two decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


