Question: The Correct answer is B 16) Consider the equilibrium system: N2O4(g)=2NO2(g) for which Kp=0.1134 at 25C and rH= 58.03kJ/mol. Assume that 1 mole of N2O4
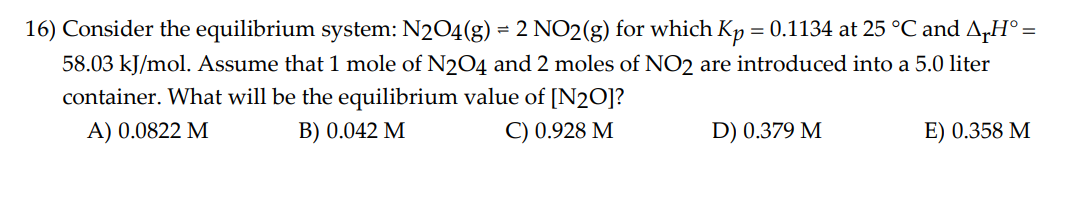 The Correct answer is B
The Correct answer is B
16) Consider the equilibrium system: N2O4(g)=2NO2(g) for which Kp=0.1134 at 25C and rH= 58.03kJ/mol. Assume that 1 mole of N2O4 and 2 moles of NO2 are introduced into a 5.0 liter container. What will be the equilibrium value of [N2O] ? A) 0.0822M B) 0.042M C) 0.928M D) 0.379M E) 0.358M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


