Question: The correct values obtained from the previous part A and part B, for [HPO4^2-] and [H2PO4^-] respectively were: 0.16 M and 0.26 M under the

![for [HPO4^2-] and [H2PO4^-] respectively were: 0.16 M and 0.26 M under](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f84ca0a1d5c_57666f84ca038aba.jpg)
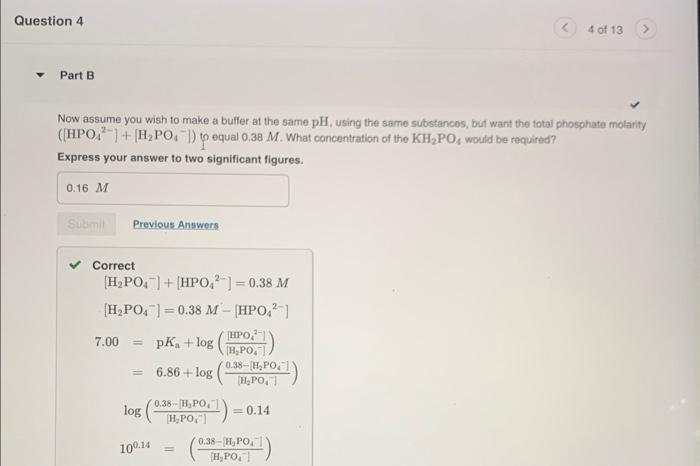
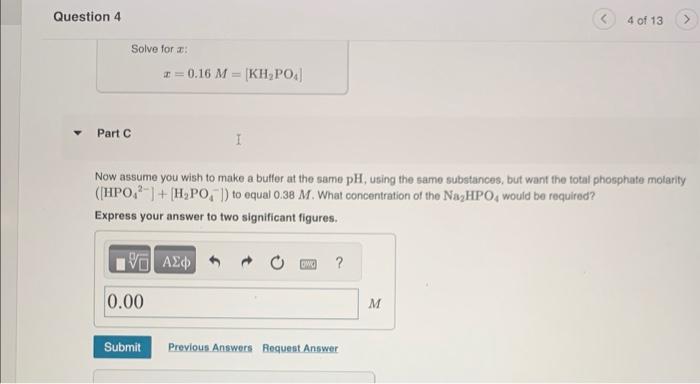
Now assume you wish to make a buffer at the same pH, using the same substances, but want the total phosphate molarity ([HPO42]+[H2PO4])to equal 0.38M. What concentration of the Na2HPO4 would be required? Express your answer to two significant figures. Suppose you wanted to make a buffer of exactly pH 7.00 using KH2PO4 and Na2HPO4. If the final solution was 0.19 M in KH2PO4, what concentration of Na2HPO4 would you need? (pK4 for H3PO4,H2PO4, and HPO42 are 2.14, 6.86, and 12.40, respectively.) Express your answer to two significant figures. CorrectH2PO4+OHHPO42+H2OpKKa=6.86pH=7.00[H2PO4]=0.19MpH=pa+log(HAA)=7.00 Now assume you wish to make a buffer at the same pH, using the same substances, but want the fotai phosphate molarity ([HPO42]+[H2PO4])to equal 0.38M. What concentration of the KH2PO4 would be required? Express your answer to two significant figures. Correct[H2PO4]+[HPO42]=0.38M[H2PO4]=0.38M[HPO42]7.00=pKa+log([H2PO4][HPO42])=6.86+log([H2PO4]0.38[H2PO4])log([H2PO4]0.38[H2PO4])=0.14100.14=([H2PO4]0.38[H2PO4]) tion 4 4 of 13 Solve for x : x=0.16M=[KH2PO4] Part C Now assume you wish to make a butfer at the same pH, using the same substances, but want the total phosphate molarity ([HPO42]+[H2PO4])to equal 0.38M. What concentration of the Na2HPO4 would be required? Express your answer to two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


