Question: The data in the table below were obtained for the reaction: 2 X + 1/2 Y + 2 Z Experiment Number [X] (M) [Y] (M)
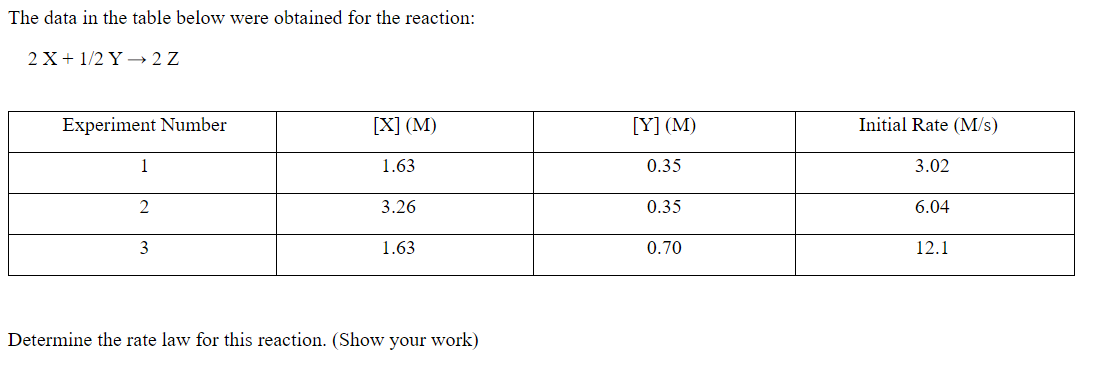
The data in the table below were obtained for the reaction: 2 X + 1/2 Y + 2 Z Experiment Number [X] (M) [Y] (M) Initial Rate (M/s) 1 1.63 0.35 3.02 2 3.26 0.35 6.04 3 1.63 0.70 12.1 Determine the rate law for this reaction. (Show your work)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


