Question: The data is shown on the second picture, the 1st conclusion questionis already answered In this experiment you will compare the heat of solution for

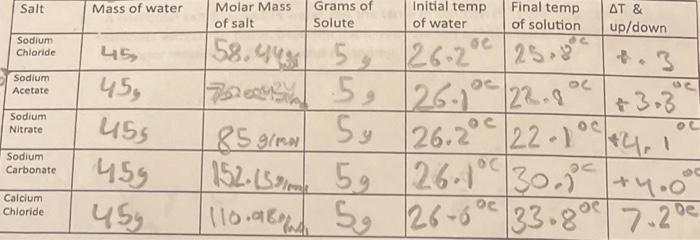

In this experiment you will compare the heat of solution for 5 different ionic compounds in order to determine the most efficient hot pack/ cold pack raw material. Remember if heat is released the surrounding temp will increase. Materials: Styrofoam calorimeter, thermometer, graduated cylinder, balance, water, sodium chloride, sodium acetate, sodium carbonate, sodium nitrate, calcium chloride Procedure: 1. Add exactly 45ml of water to the calorimeter, take the temp. Add exactly 5.0g of a salt to the water, stir and record the largest temp change. 2. Repeat with each salt. Make sure to clean the cup between trials. Each solution can go down th drain with plenty of water. Calculations: show your work 1. Calculate the heat released or absorbed for each trial (q) in Joules. Massofsolution=50g,C=4,18J/gC 2. Calculate the heat released or absorbed per gram of salt (J/g) 3. Calculate the moles of each salt used 4. Calculate H solution in kJ per mole - make sure to include the sign (+Or) NaCl2CSO 1. Write equations for the dissolving of each salt, omit water 2. Explain using evidence from your data table which salts dissolved endothermically and which dissolved exothermically. 3. Give the sign of H,G and S for each dissolving equation. Then identify the driving force for the dissolving of each salt. 4. Which salt makes the best hot pack/ cold pack? Justify. 5. A particular salt dissolves in water and the temp of solution rises 2.0C/g. Where do the stronger attractions occur, between the ions in the salt or between the dissolved ions and the water molecules? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


