Question: the data table below. Data Table Vapor Pressure (Torr) isoo Torr Temperature (K) 393.38K 3. The Clausius-Clapeyron equation may be written in several forms. For
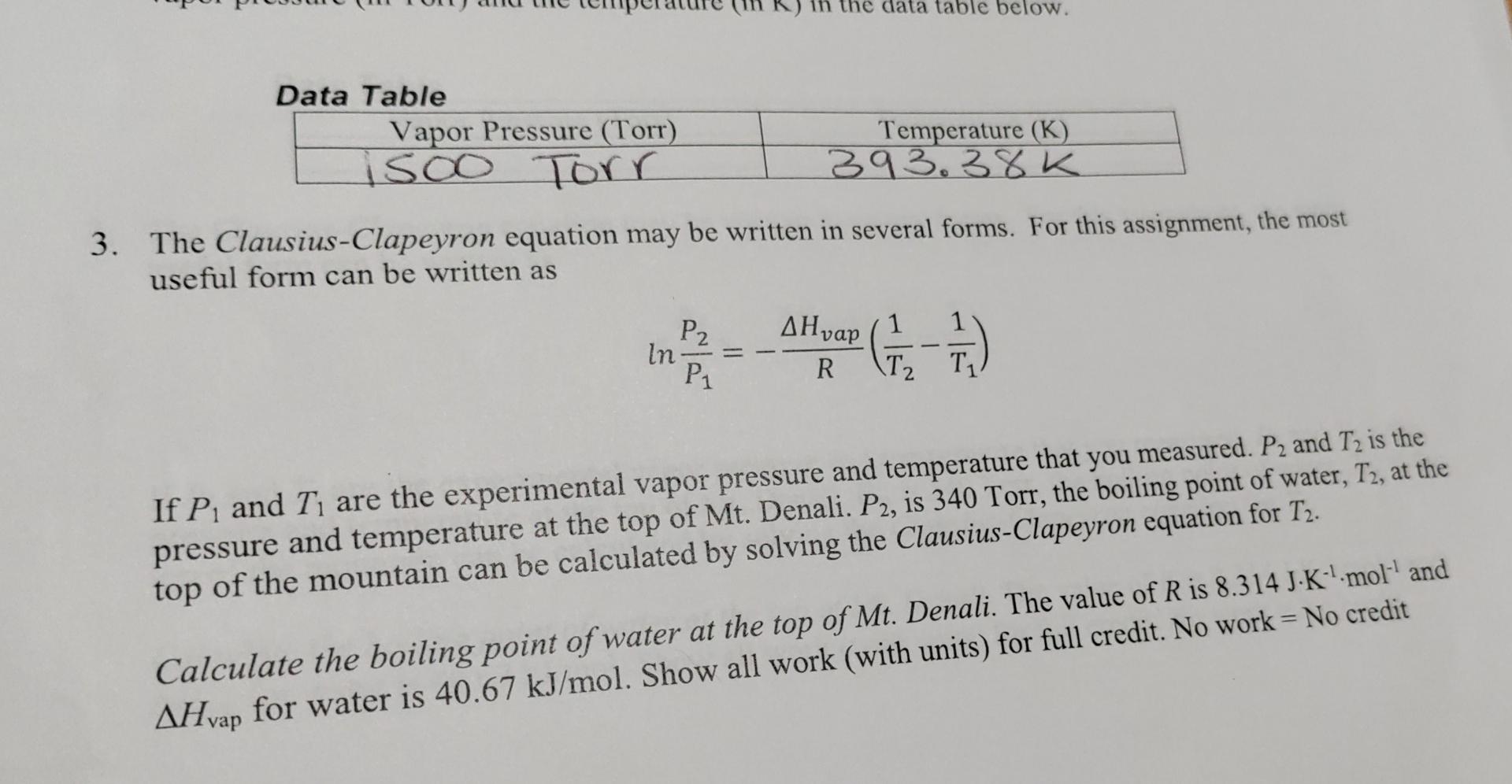

the data table below. Data Table Vapor Pressure (Torr) isoo Torr Temperature (K) 393.38K 3. The Clausius-Clapeyron equation may be written in several forms. For this assignment, the most useful form can be written as AHvap P2 In P1 () = R \T2 If P, and T, are the experimental vapor pressure and temperature that you measured. P2 and T2 is the pressure and temperature at the top of Mt. Denali. P2, is 340 Torr, the boiling point of water, T2, at the top of the mountain can be calculated by solving the Clausius-Clapeyron equation for T2. Calculate the boiling point of water at the top of Mt. Denali. The value of R is 8.314 J-K-L-mol- and AHvap for water is 40.67 kJ/mol. Show all work (with units) for full credit. No work = No credit Point of Water at High Altitude The relationship between the equilibrium vapor pressure of a liquid or solid and temperature is given by the Clausius-Clapeyron equation. In this assignment you will measure the vapor pressure of water at a given temperature and use this data and the Clausius-Clapeyron equation to calculate the boiling point of water on the top of Mt. Denali in Alaska. 1. To start this activity, click the icon for Activities on the left side of the screen (BeyondLabz Web). Enter Boiling Point of Water at High Altitude into the search window. Instructions on how to gain access to the experiment will be listed along with a link that will take you to the lab. The lab will load in a new tab. 2. The balloon is filled with 0.40 moles of water vapor at a pressure of 1500 Torr and a temperature of 400 K. Click the down arrows in the Live Data Temperature control area until the temperature stops decreasing. This temperature represents the equilibrium temperature where water as a gas exists in equilibrium with water as a liquid. The pressure at this temperature is the vapor pressure. Record the vapor pressure (in Torr) and the temperature (in K) in the data table below. Data Table Vapor Pressure (Torr) isoo Torr Temperature (K) 393.38K 3. The Clausius-Clapeyron equation may be written in several forms. For this assignment, the most useful form can be written as 1 P2 In Go R T2 T If P, and Ti are the experimental vapor pressure and temperature that you measured. P, and T2 is the pressure and temperature at the top of Mt. Denali. P2, is 340 Torr, the boiling point of water, T3, at the top of the mountain can be calculated by solving the Clausius-Clapeyron equation for T2. Calculate the boiling point of water at the top of Mt. Denali. The value of R is 8.314 J-K--mol-' and AHvap for water is 40.67 kJ/mol. Show all work (with units) for full credit. No work = No credit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


