Question: The data you have obtained from the table in the first question is at 298K and 1 bar. However, human body temperature, which we will


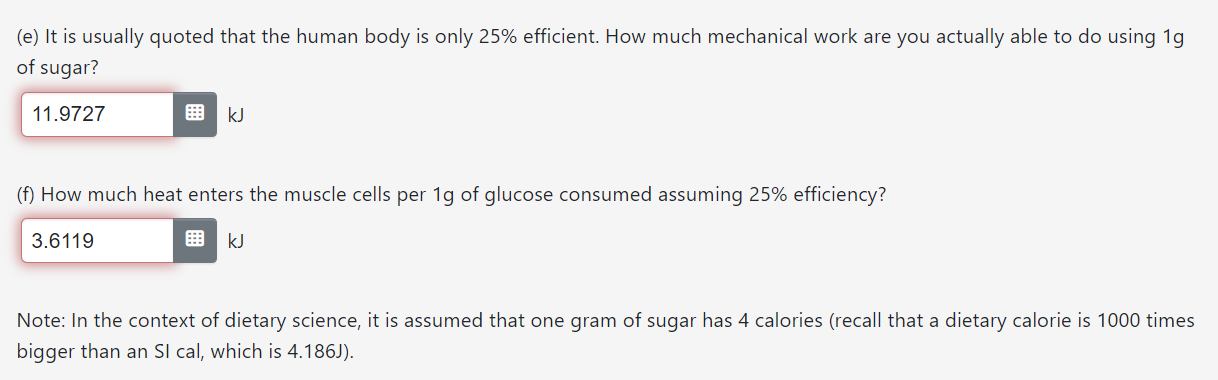



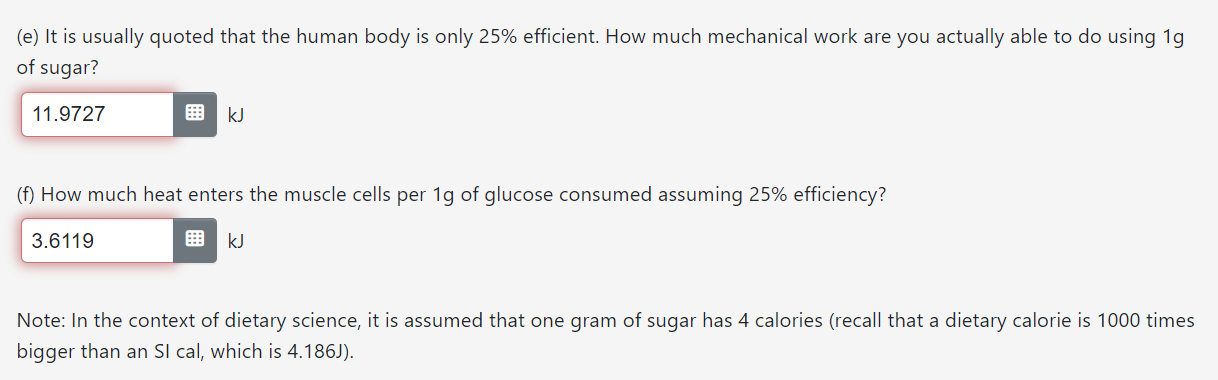

The data you have obtained from the table in the first question is at 298K and 1 bar. However, human body temperature, which we will take to be 37C, is about 12 degrees warmer than 298K. Before we continue with the calculations, we will need to correct the data for this temperature difference. (a) How much does AG for the reaction (per 1g of glucose) change between 25C and 37C? (b) How much does A3 for the reaction (per 1g of glucose) change between 25C and 37C? Hint: changes in entropy as the temperature is raised can be related to the heat capacity at constant pressure. UK (c) How much does AH for the reaction (per 1g of glucose) change between 25C and 37C? The data you have obtained from the table in the first question is at 298K and 1 bar. However, human body temperature, which we will take to be 37C, is about '12 degrees warmer than 298K. Before we continue with the calculations, we will need to correct the data for this temperature difference. (a) How much does AG for the reaction (per 19 of glucose) change between 25C and 37C? Hint: One of the derivatives in Worksheet? should be useful. -0.0163 k] (b) How much does A3 for the reaction (per 19 of glucose) change between 25C and 37C? Hint: changes in entropy as the temperature is raised can be related to the heat capacity at constant pressure. 0.0839 J/K (c) How much does AH for the reaction (per 19 of glucose) change between 25C and 37C? Hint: There are two ways to do this: use another derivative from Worksheet 7, or use the relationship between G and H (but be careful, temperature is changing). 0.026 kJ (e) It is usually quoted that the human body is only 25% efficient. How much mechanical work are you actually able to do using 1g of sugar? 11 .9?27 E k] (0 How much heat enters the muscle cells per 1g of glucose consumed assuming 25% efficiency? 3.6119 R kJ Note: In the context of dietary science, it is assumed that one gram of sugar has 4 calories (recall that a dietary calorie is 1000 times bigger than an SI cal, which is 4.186J). Continuing from the previous question, compute the quantities below per 1 gram of glucose under conditions of 1atm and human body temperature. (a) Energy available, i.e. the total amount of energy that burning/oxidizing glucose can deposit into the environment l 155846 I k] (b) How much mechanical work should you be able to do using 1g of sugar, if your body operated reversibly? l 159636 I k] (c) How much heat enters the muscle cells per 1g of glucose consumed assuming reversible operation? 0.4213 E k] (d) What is the ideal efficiency of glucose oxidation (efficiency 2 benefit/cost = work/energy available)? l 1.0243
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


