Question: The electron configuration shown below represents the ground-state electron configuration for an atom of which element? [Kr]4d105s1 Choose one: cadmium, Cd silver, Ag rubidium, Rb
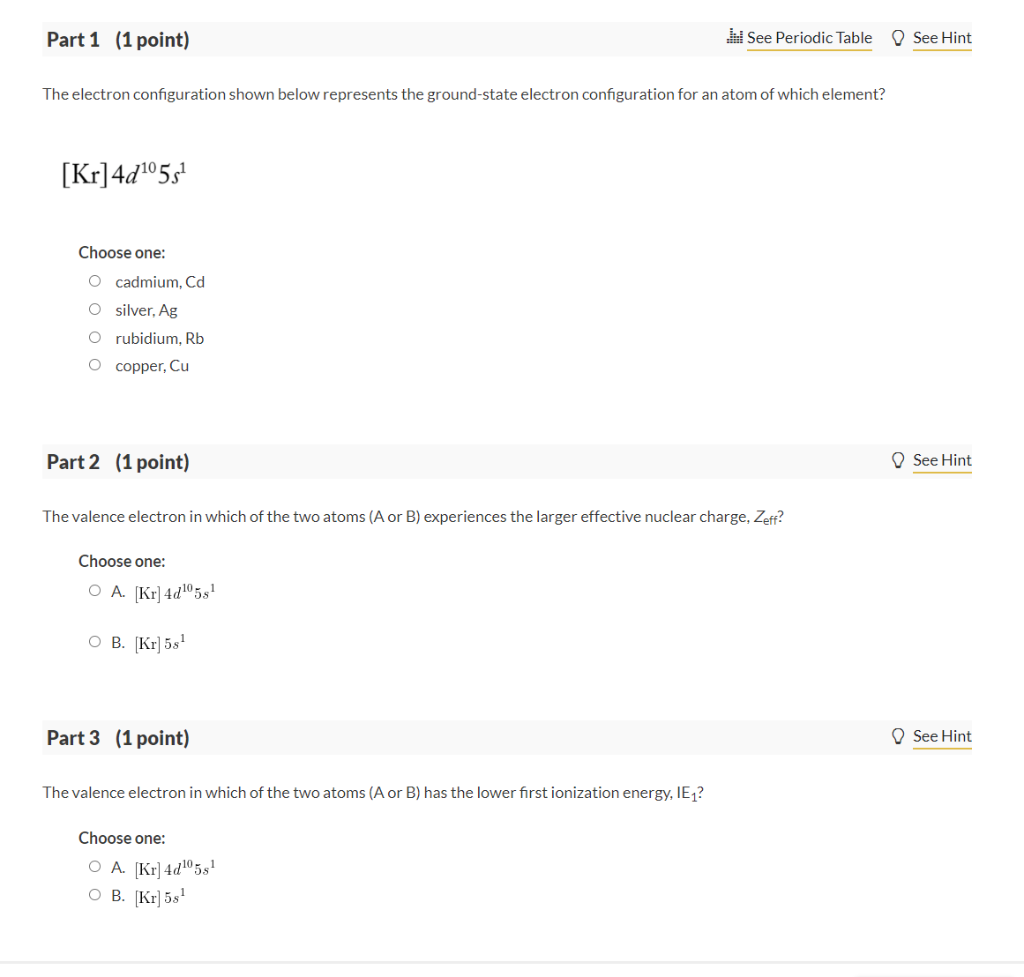
The electron configuration shown below represents the ground-state electron configuration for an atom of which element? [Kr]4d105s1 Choose one: cadmium, Cd silver, Ag rubidium, Rb copper, Cu Part 2 (1 point) The valence electron in which of the two atoms (A or B) experiences the larger effective nuclear charge, Zeff ? Choose one: A. [Kr]4d105s1 B. [Kr]5s1 Part 3 (1 point) The valence electron in which of the two atoms (A or B ) has the lower first ionization energy, IE1 ? Choose one: A. [Kr]4d105s1 B. [Kr]5s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


