Question: The elementary and gaseous reaction, 2 A B + C , with specific reaction rate of 9 . 5 d m m o l g
The elementary and gaseous reaction, with specific reaction rate of cat at is carried out in a packed bed reactor PBR isothermally at with pressure drop parameter, aequals to Pure is fed at pressure of atmospheresandat temperature of and a production rate of of is required.
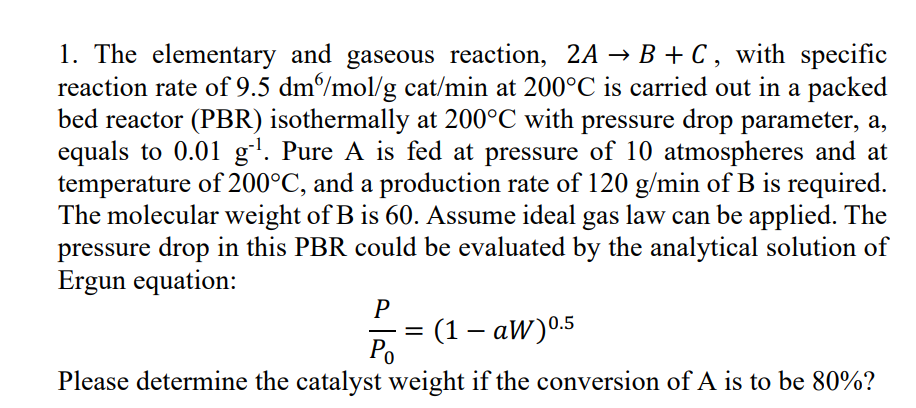
The molecular weight of B is Assume ideal gas law can be applied. The pressure drop in this PBR could be evaluated by the analytical solution of Ergun equation:
Please determine the catalyst weight if the conversion of A is to be
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


