Question: The elementary reaction 2H2O(g)----2H2(g) + O2(g) proceeds at a certain temperature until the partial pressures of H2O, H2, and O2 reach 0.0750 atm, 0.00700
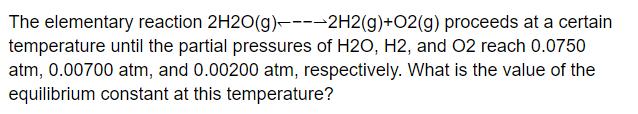
The elementary reaction 2H2O(g)----2H2(g) + O2(g) proceeds at a certain temperature until the partial pressures of H2O, H2, and O2 reach 0.0750 atm, 0.00700 atm, and 0.00200 atm, respectively. What is the value of the equilibrium constant at this temperature?
Step by Step Solution
3.29 Rating (164 Votes )
There are 3 Steps involved in it
The equilibrium constant for this reaction at the given tempera... View full answer

Get step-by-step solutions from verified subject matter experts


