Question: the expression: EN=rC+Dexp(r) Where r is the interionic separation and C, D, and are constants whose values depend on the specific material. (i) Derive an
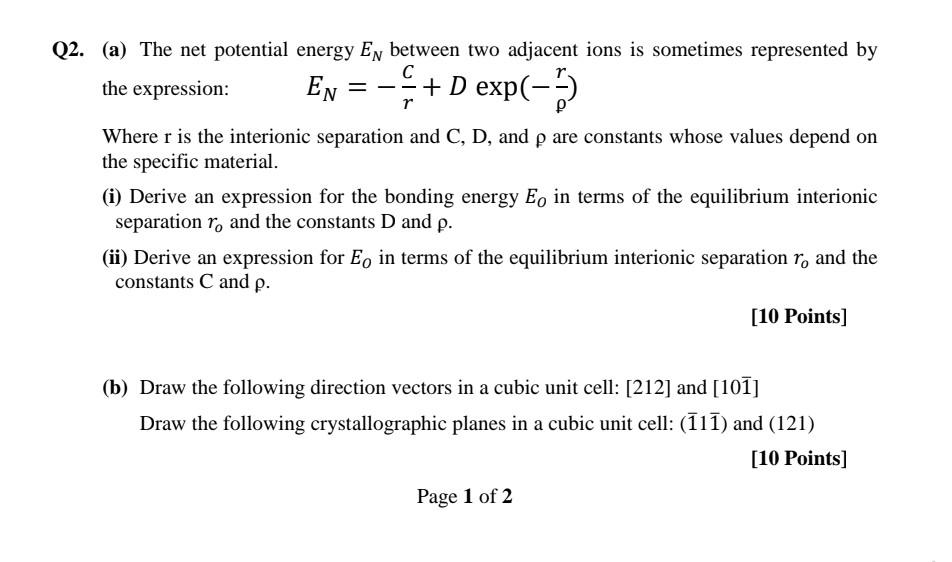
the expression: EN=rC+Dexp(r) Where r is the interionic separation and C, D, and are constants whose values depend on the specific material. (i) Derive an expression for the bonding energy EO in terms of the equilibrium interionic separation ro and the constants D and . (ii) Derive an expression for EO in terms of the equilibrium interionic separation ro and the constants C and . [10 Points] Draw the following crystallographic planes in a cubic unit cell: (111) and (121)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


