Question: - The first step in processing zinc metal from its ore, Zn, is to react it with O, according to the reaction 2 ZnS(s) +
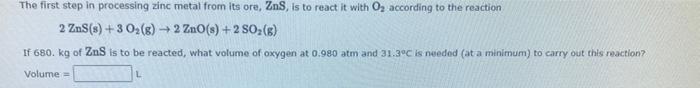
- The first step in processing zinc metal from its ore, Zn, is to react it with O, according to the reaction 2 ZnS(s) + 3 O2(g) + 2 ZnO(s) +2 SO (6) If 680 kg of ZnS is to be reacted, what volume of oxygen at 0.980 atm and 31.3C is needed (at a minimum) to carry out this reaction Volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


