Question: The following compounds are stereoisomers. Rank in order of increasing the stability (the least stable to the most stable.) CI CI CI CI A. B.
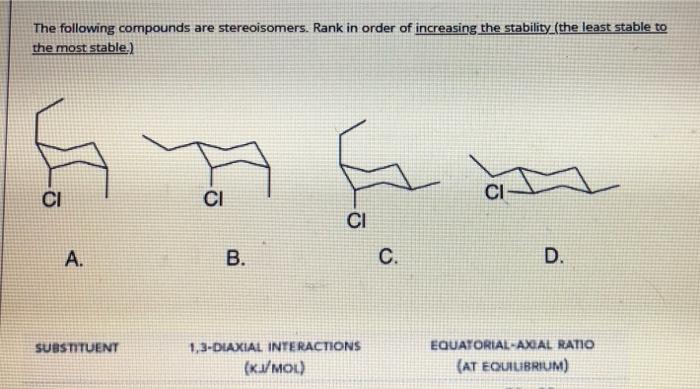
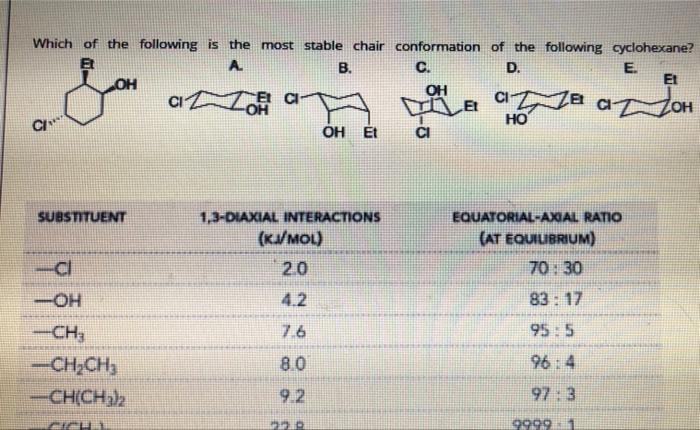
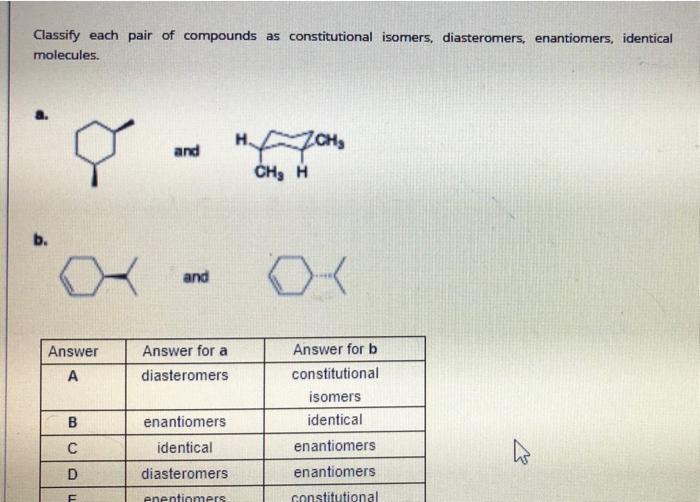
The following compounds are stereoisomers. Rank in order of increasing the stability (the least stable to the most stable.) CI CI CI CI A. B. C. D. SUBSTITUENT 1,3-DIAXIAL INTERACTIONS (KJ/MOL) EQUATORIAL-AXIAL RATIO (AT EQUILIBRIUM) Which of the following is the most stable chair conformation of the following cyclohexane? . B. C. D. E OH OH HO OH Et So anOC a La aa a oH C SUBSTITUENT 1,3-DIAXIAL INTERACTIONS (KVMOU) EQUATORIAL-AXIAL RATIO (AT EQUILIBRIUM) 70. 30 ci 2.0 -OH 4.2 83: 17 95.5 7.6 -CH, -CH2CH3 -CH(CH3)2 8.0 964 92 97: 3 999911 Classify each pair of compounds as constitutional isomers, diasteromers, enantiomers, identical molecules. and , , b. and OK Answer Answer for a diasteromers A Answer for b constitutional isomers identical enantiomers B enantiomers identical diasteromers D enantiomers constitutional F enentiomers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


