Question: The following data was obtained from an initial solution containing methyl iodide (MI) and dimethyl-p-toluidine (PT), both concentrations of 0.05 mol/L. The equilibrium constant of
The following data was obtained from an initial solution containing methyl iodide (MI) and dimethyl-p-toluidine (PT), both concentrations of 0.05 mol/L. The equilibrium constant of the reaction is 1.43, under the conditions at which the kinetic data were taken.
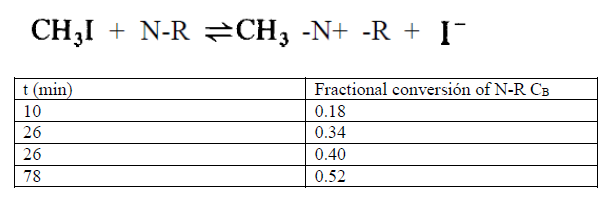
Using this information, we are asked to determine the kinetic model, indicating the values of the direct and inverse kinetic constants.
CHZI + N-R =CH2 -N+ -R + I- t (min) 10 26 Fractional conversion of N-R CB 0.18 0.34 0.40 0.52 26 78
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


