Question: The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not
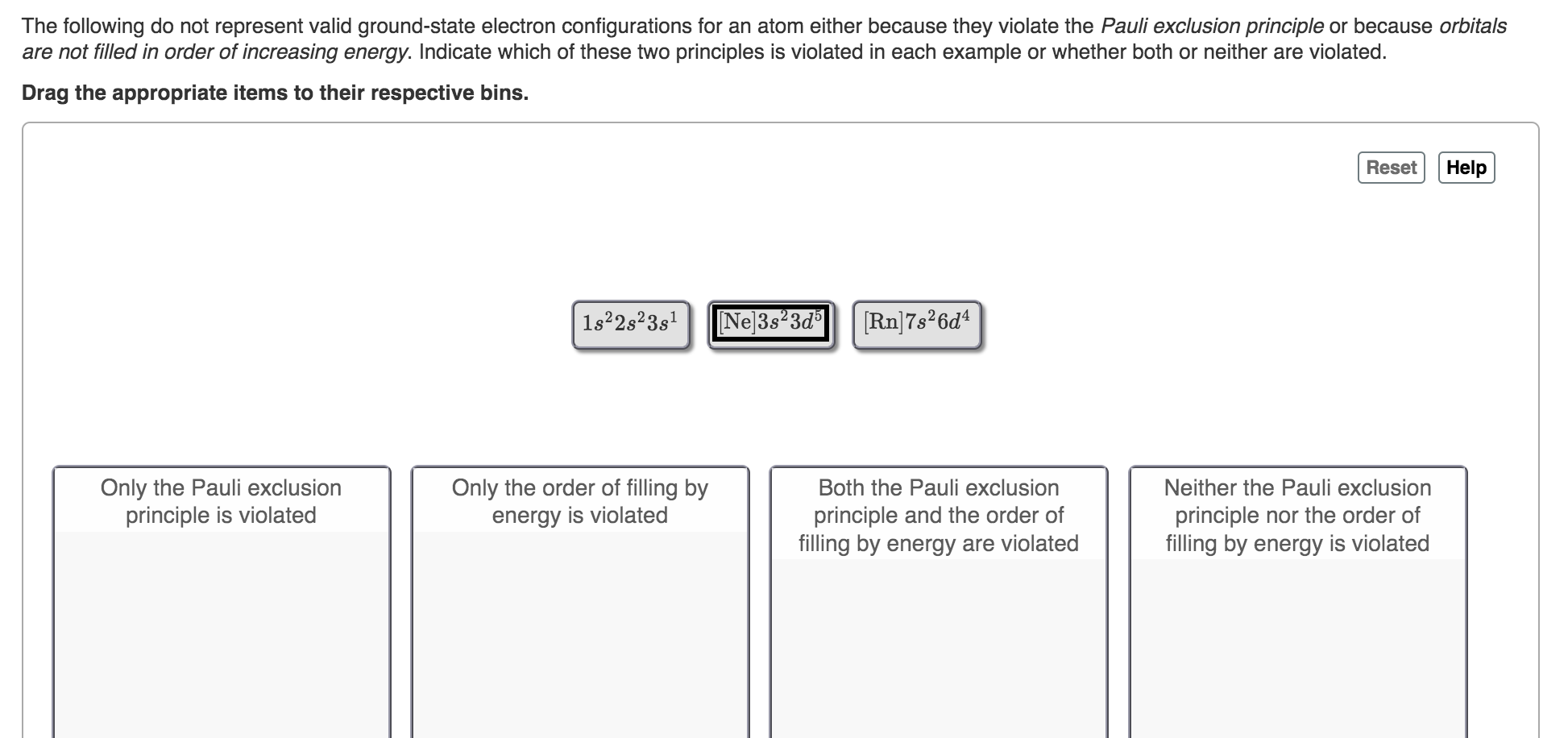
The following do not represent valid ground-state electron configurations for an atom either because they violate the Pauli exclusion principle or because orbitals are not filled in order of increasing energy. Indicate which of these two principles is violated in each example or whether both or neither are violated. Drag the appropriate items to their respective bins
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


