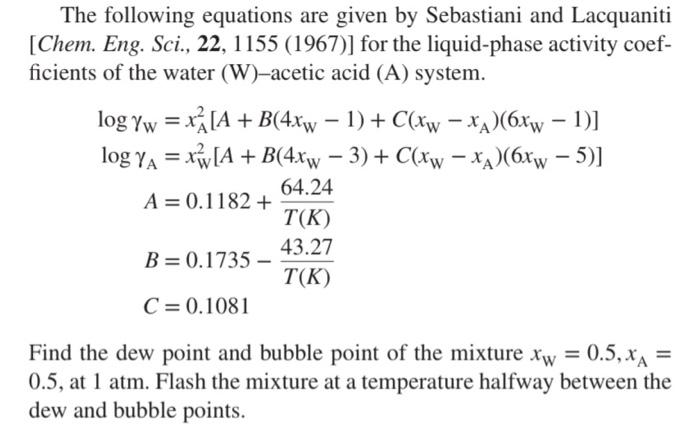
Question: = = - The following equations are given by Sebastiani and Lacquaniti [Chem. Eng. Sci., 22, 1155 (1967)] for the liquid-phase activity coef- ficients of
![Lacquaniti [Chem. Eng. Sci., 22, 1155 (1967)] for the liquid-phase activity coef-](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d57f234ba_61466f8d57ec6d09.jpg)
= = - The following equations are given by Sebastiani and Lacquaniti [Chem. Eng. Sci., 22, 1155 (1967)] for the liquid-phase activity coef- ficients of the water (W)-acetic acid (A) system. log yw = x [A + B(4xw 1) + C(Xw xx)(6Xw 1)] log A =x*(A + B(4x - 3)+ C(Xw -x2(6xx - 5)] 64.24 A = 0.1182 + T(K) 43.27 B=0.1735 - T(K) C = 0.1081 - Find the dew point and bubble point of the mixture xw = 0.5, xa = 0.5, at 1 atm. Flash the mixture at a temperature halfway between the dew and bubble points. The final step asks you to flash the mixture at a particular temperature: Determine V/F and the compositions in the two phases. To complete this problem, you will need to find equations that relate the vapor pressure and temperature for water and acetic acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


