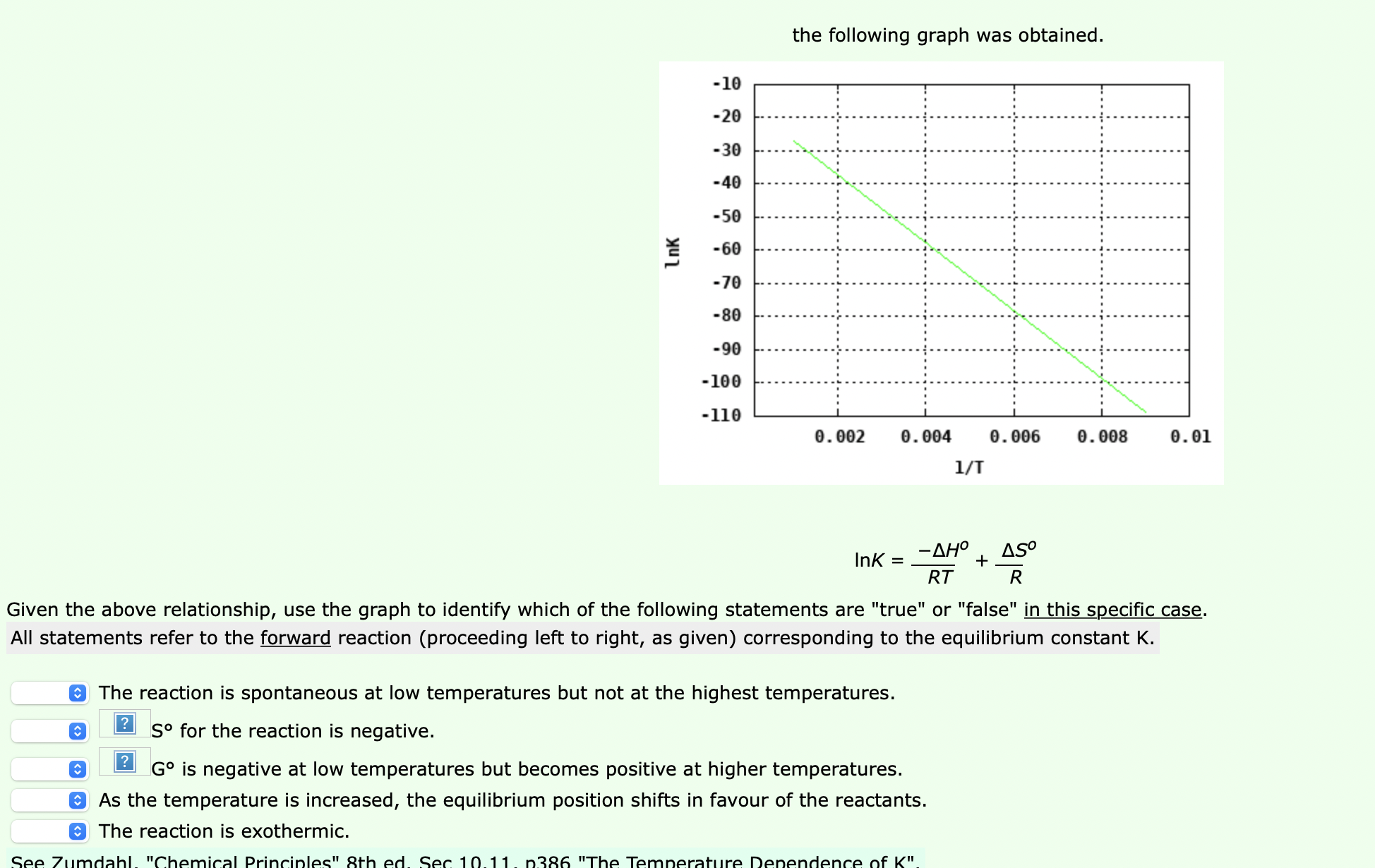
Question: the following graph was obtained. l n K = - H 0 R T + S o R Given the above relationship, use the graph
the following graph was obtained.
Given the above relationship, use the graph to identify which of the following statements are "true" or "false" in this specific case.
All statements refer to the forward reaction proceeding left to right, as given corresponding to the equilibrium constant
The reaction is spontaneous at low temperatures but not at the highest temperatures.
for the reaction is negative.
is negative at low temperatures but becomes positive at higher temperatures.
As the temperature is increased, the equilibrium position shifts in favour of the reactants.
The reaction is exothermic.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


