Question: The following pair HCN/KCN can be considered as a buffer solution* 2 points True False Ka (Acidity Constant) depends on: 3 points Temperature % dissociation
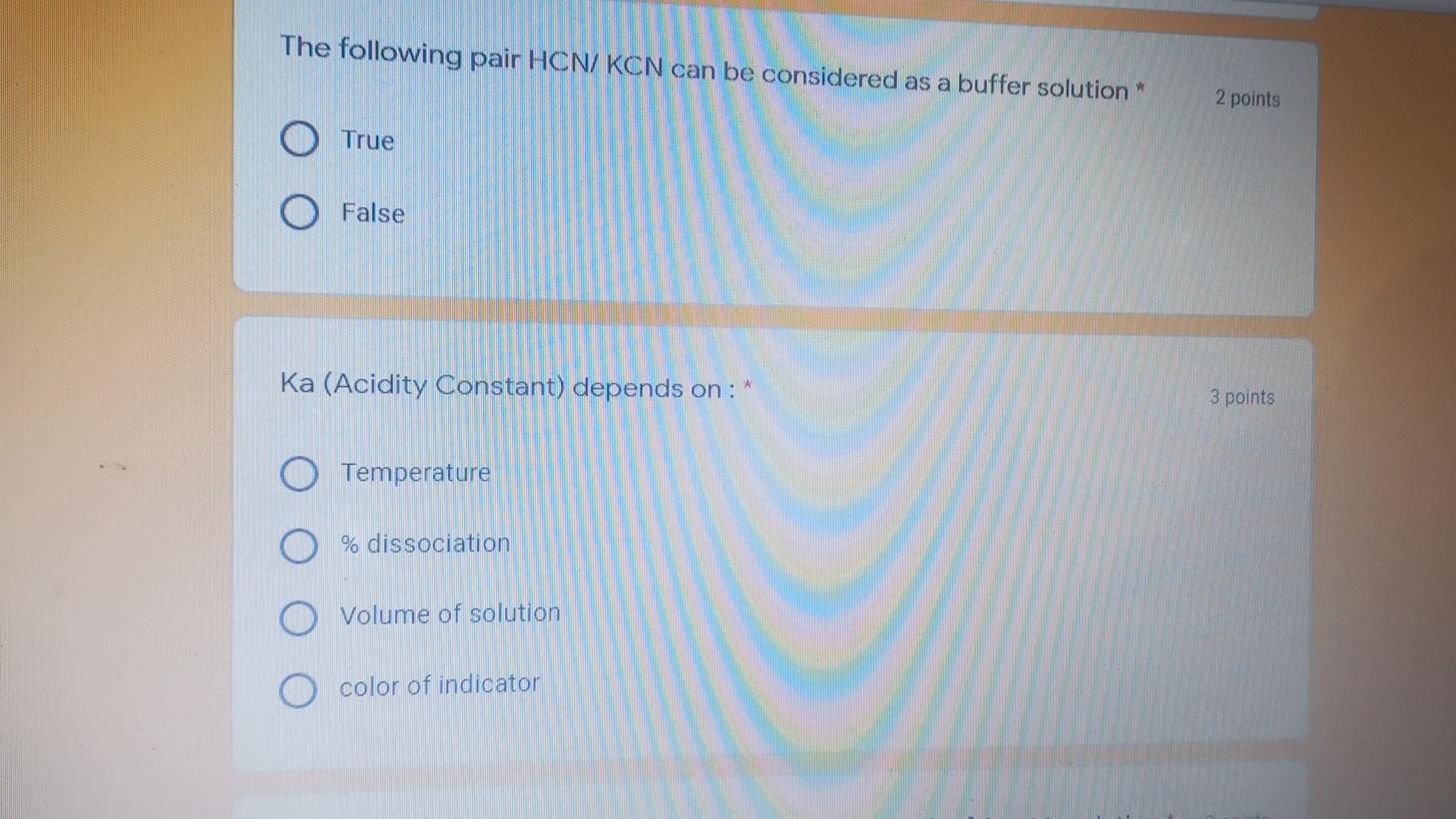
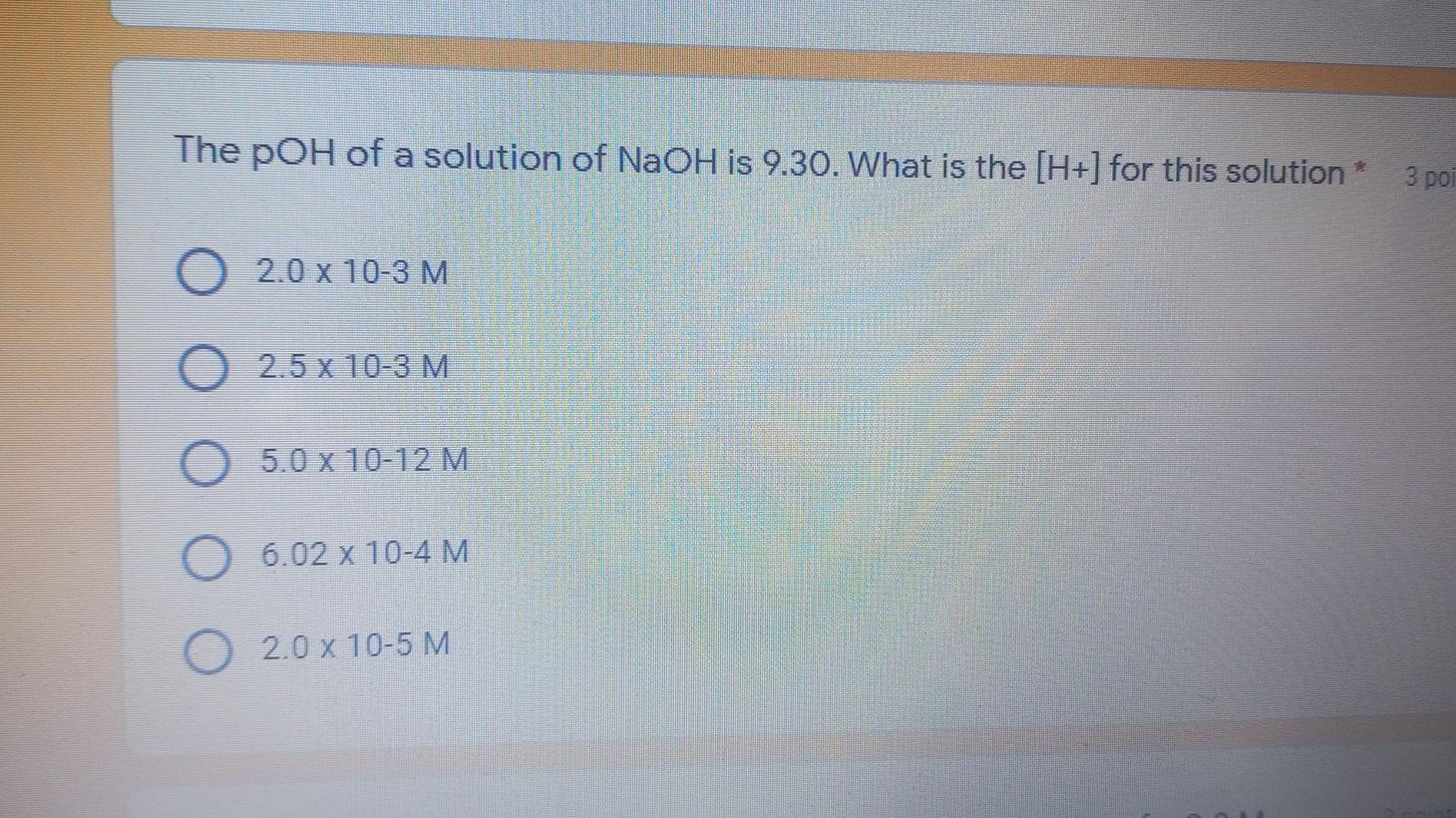
The following pair HCN/KCN can be considered as a buffer solution* 2 points True False Ka (Acidity Constant) depends on: 3 points Temperature % dissociation Volume of solution color of indicator The poH of a solution of NaOH is 9.30. What is the [H+] for this solution* 3 poi 2.0 x 10-3M 2.5 x 10-3 M. 5.0 x 10-12 M 6.02 x 10-4 M 2.0 x 10-5 M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


