Question: The following table list the specific heat for different substances including water. Table 14.1Specfic Heats of Some Fam kar Substances at 25 Substance Specific
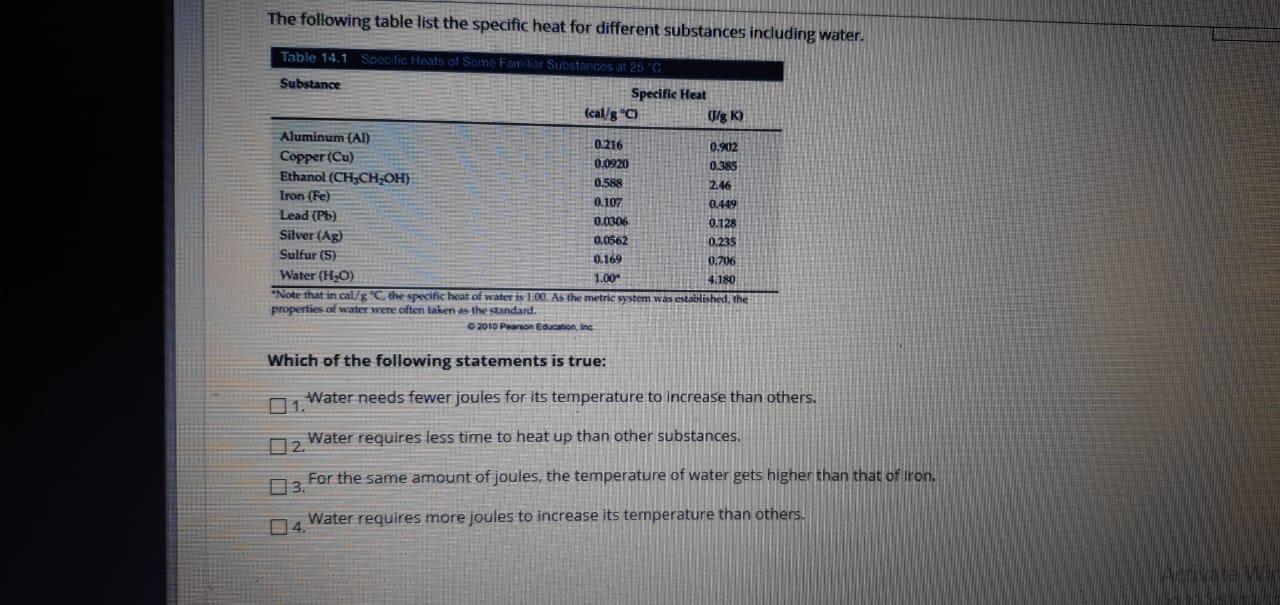
The following table list the specific heat for different substances including water. Table 14.1Specfic Heats of Some Fam kar Substances at 25 Substance Specific Heat (cal/g "C) (/g K) Aluminum (AI) 0.216 0.902 Copper (Cu) Ethanol (CH,CH;OH} 0.0920 0.385 2.46 0.588 Iron (Fe) 0.107 0.449 Lead (Pb) 0.0306 0.128 Silver (Ag) 0.0562 0.235 Sulfur (S) 0.169 0.706 Water (HO) 1.00 4.180 *Note that in cal/g "C the specific heat of water is 100. As the metric system was established, the properties of water were often taken as the standard. 2010 Pearson Education, Inc Which of the following statements is true: Water needs fewer joules for its temperature to increase than others. 1. 02. Water requires less time to heat up than other substances. For the same amount of joules, the temperature of water gets higher than that of Iron. 3. Water requires more joules to increase its termperature than others. 4.
Step by Step Solution
3.30 Rating (150 Votes )
There are 3 Steps involved in it
Specific heat of a substance is basically the energy required to increase ... View full answer

Get step-by-step solutions from verified subject matter experts


