Question: The following two reactions occur simultaneously in a batch reactor: CH4 (g) + H2O(g) CO(g) + 3H2(9) CO(g) + H2O(g) + CO2(g) + H2(g) A
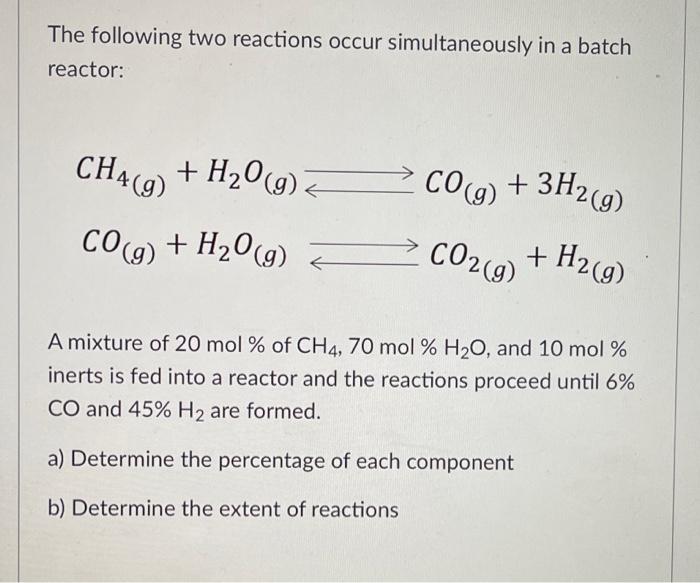
The following two reactions occur simultaneously in a batch reactor: CH4 (g) + H2O(g) CO(g) + 3H2(9) CO(g) + H2O(g) + CO2(g) + H2(g) A mixture of 20 mol % of CH4, 70 mol % H2O, and 10 mol % inerts is fed into a reactor and the reactions proceed until 6% CO and 45% H2 are formed. a) Determine the percentage of each component b) Determine the extent of reactions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


