Question: The formal charge is the charge an element would have in a molecule or ion if all of the bonding electrons were shared equally
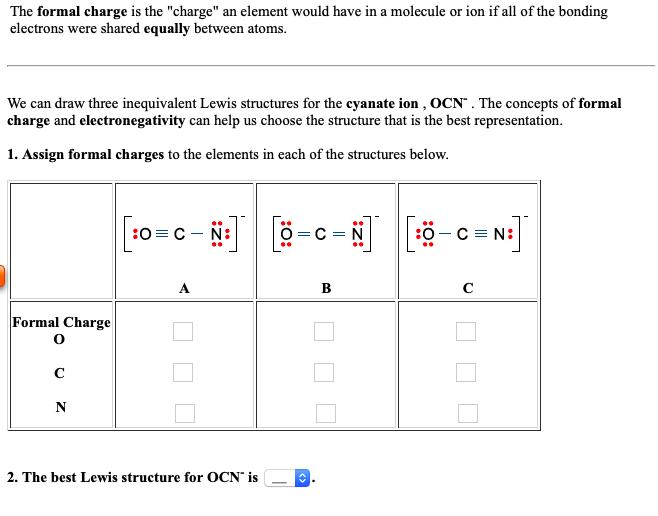
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms We can draw three inequivalent Lewis structures for the cyanate ion , OCN. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below OC N: :03 - N: O C Formal Charge 2. The best Lewis structure for OCN is z
Step by Step Solution
3.27 Rating (147 Votes )
There are 3 Steps involved in it
To assign formal charges and determine the best Lewis structure for the cyanate ion textOCN lets fol... View full answer

Get step-by-step solutions from verified subject matter experts


