Question: The formula is valid for 25C. The default potential is Eoo is given for two of the reactions. The values for 500 for the other
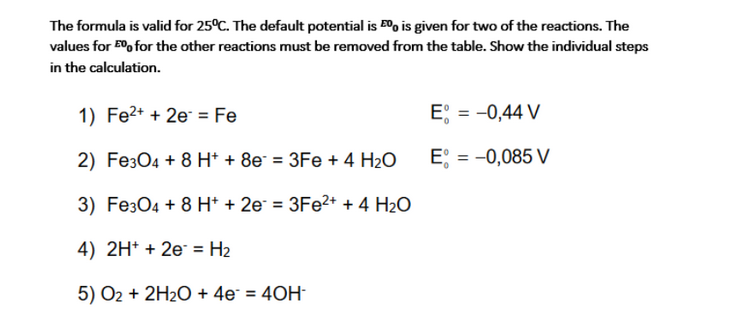
The formula is valid for 25C. The default potential is Eoo is given for two of the reactions. The values for 500 for the other reactions must be removed from the table. Show the individual steps in the calculation. 1) Fe2+ + 2e = Fe E = -0,44 V 2) Fe3O4 + 8 H+ + 8e = 3Fe + 4 H2O E = -0,085 V : 3) Fe3O4 + 8 H+ + 2e = 3Fe2+ + 4 H2O 4) 2H+ + 2e = H2 5) O2 + 2H2O + 4e* = 40H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


