Question: The half - life for the ( first - order ) radioactive decay of ? 1 4 C is 5 7 3 0 y .
The halflife for the firstorder radioactive decay of is An archaeological sample contained wood that had only per cent of the found in living trees. What is its age?
Nitrogen gas adsorbed on charcoal to the extent of at kPa and but at the same amount of adsorption was achieved only when the pressure was increased to MPa. What is the enthalpy of adsorption of nitrogen on charcoal?
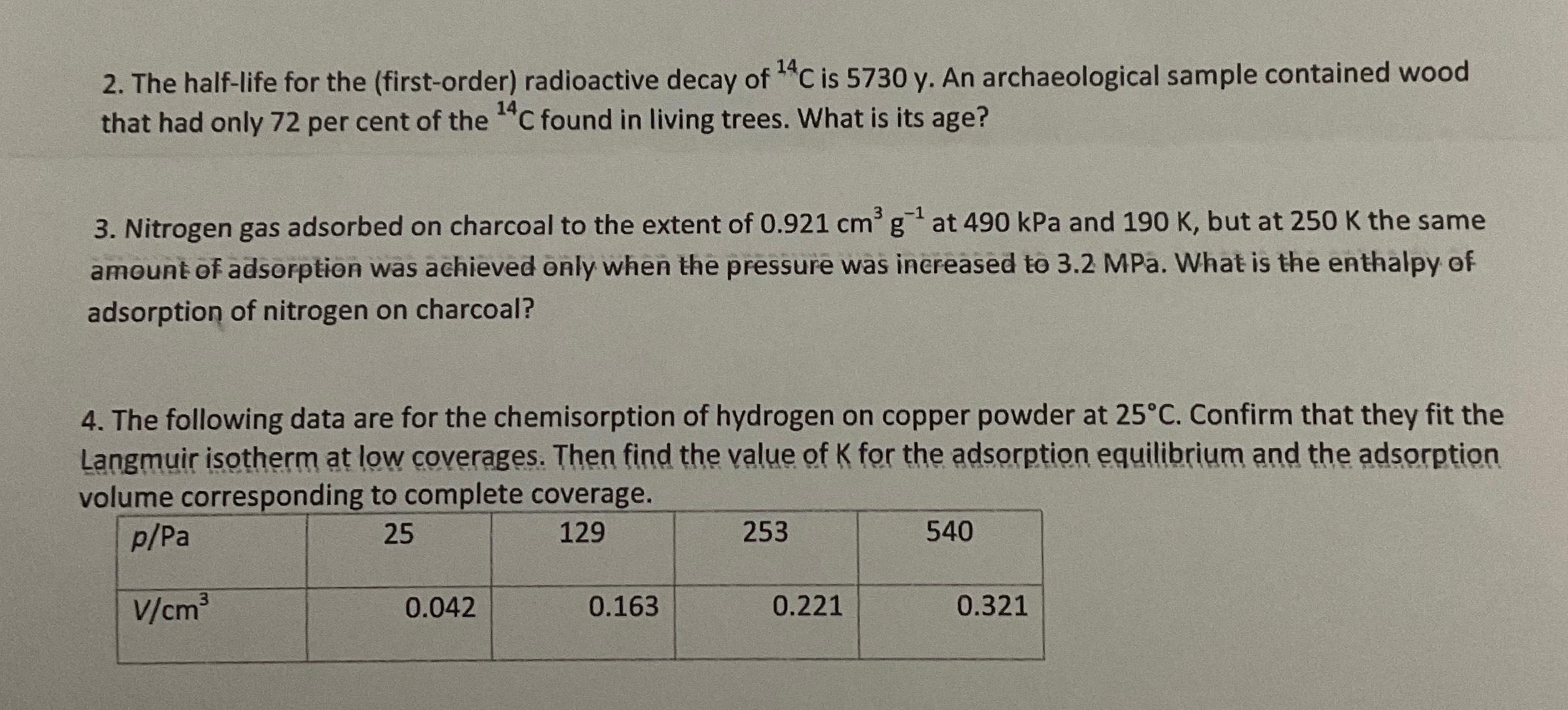
The following data are for the chemisorption of hydrogen on copper powder at Confirm that they fit the Langmuir isotherm at low coverages. Then find the value of for the adsorption equilibrium and the adsorption volume corresponding to complete coverage.
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


