Question: The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight line,
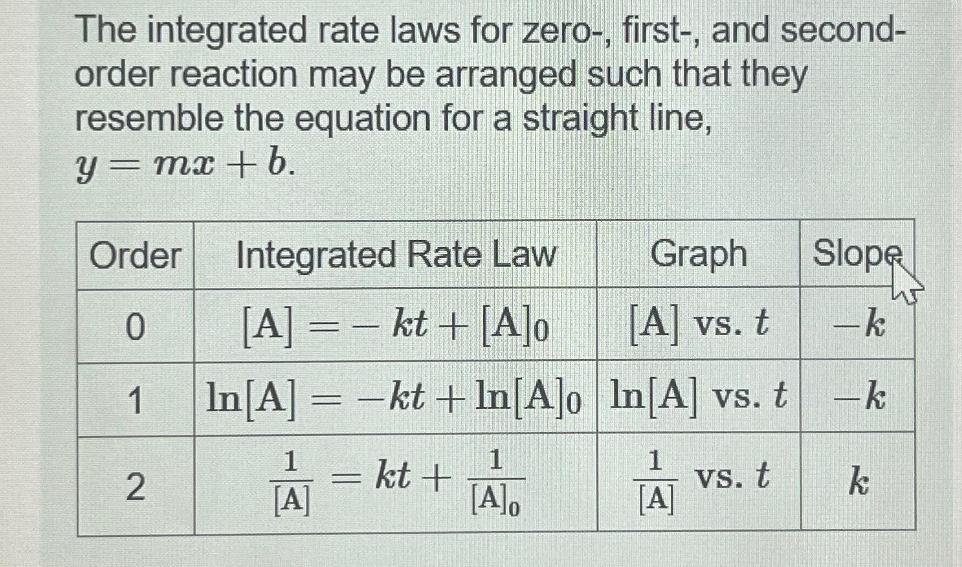
The integrated rate laws for zero-, first-, and second- order reaction may be arranged such that they resemble the equation for a straight line, = mx + b. y = Order 0 1 2 Integrated Rate Law [A] = - kt + [A]o In [A] = -kt+In[A]o 1 [A] - kt+ 1 [A]o Graph [A] vs. t In[A] vs. t 1 vs. t [A] Slope -k -k k
Step by Step Solution
There are 3 Steps involved in it
A A2 kt2t1 A1 150102 k300135 8102 k 394104 Ms B ... View full answer

Get step-by-step solutions from verified subject matter experts


