Question: the last question should be solved by using matrix Question 0.3. Sodium hydroxide (NaOH, also known as lye, or caustic soda, because it is highly
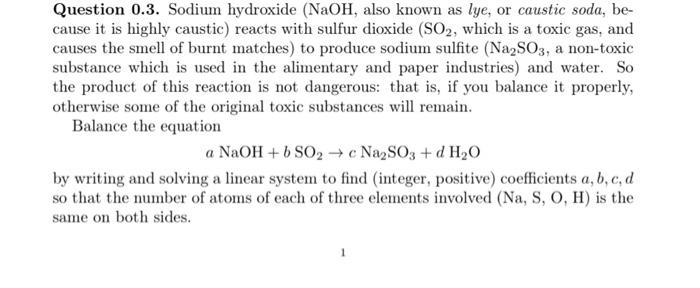
Question 0.3. Sodium hydroxide (NaOH, also known as lye, or caustic soda, because it is highly caustic) reacts with sulfur dioxide (SO2, which is a toxic gas, and causes the smell of burnt matches ) to produce sodium sulfite (Na2SO3, a non-toxic substance which is used in the alimentary and paper industries) and water. So the product of this reaction is not dangerous: that is, if you balance it properly, otherwise some of the original toxic substances will remain. Balance the equation aNaOH+bSO2cNa2SO3+dH2O by writing and solving a linear system to find (integer, positive) coefficients a,b,c,d so that the number of atoms of each of three elements involved (Na,S,O,H) is the same on both sides
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


