Question: the last two should be in scientific notation Predict and calculate the effect of volume change on an equilibrium system. Consider the equilibrium between PCl5,PCl3
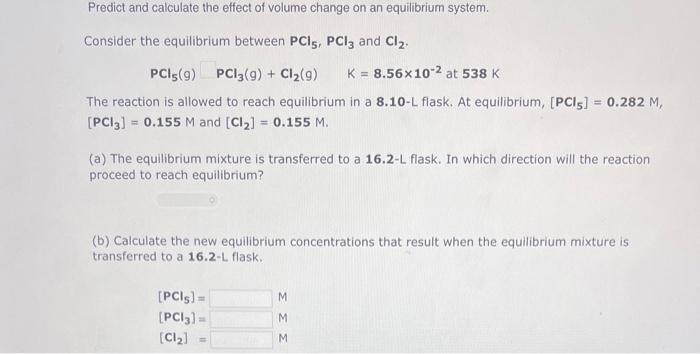
Predict and calculate the effect of volume change on an equilibrium system. Consider the equilibrium between PCl5,PCl3 and Cl2. PCl5(g)PCl3(g)+Cl2(g)K=8.56102at538K The reaction is allowed to reach equilibrium in a 8.10LL flask. At equilibrium, [PCl5]=0.282M, [PCl3]=0.155M and [Cl2]=0.155M. (a) The equilibrium mixture is transferred to a 16.2-L flask. In which direction will the reaction proceed to reach equilibrium? (b) Calculate the new equilibrium concentrations that result when the equilibrium mixture is transferred to a 16.2-L flask. [PCl5]=[PCl3]=[Cl2]=MMM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


