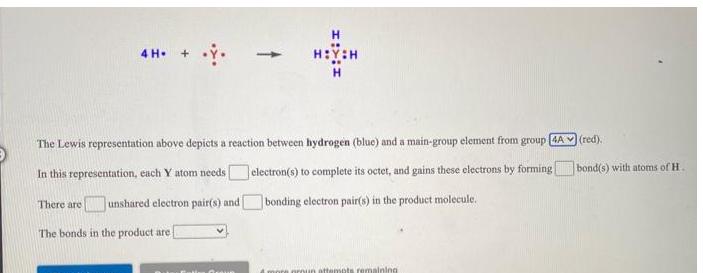
The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group...
Fantastic news! We've Found the answer you've been seeking!
Question:
Transcribed Image Text:
The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group [4A In this representation, each Y atom needs There are unshared electron pair(s) and electron(s) to complete its octet, and gains these electrons by forming bonding electron pair(s) in the product molecule. The bonds in the product are 4 more group attempts remaining (red). bond(s) with atoms of H. The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group [4A In this representation, each Y atom needs There are unshared electron pair(s) and electron(s) to complete its octet, and gains these electrons by forming bonding electron pair(s) in the product molecule. The bonds in the product are 4 more group attempts remaining (red). bond(s) with atoms of H.
Expert Answer:
Related Book For 

Posted Date:
Students also viewed these chemical engineering questions
-
Group 4A elements have much more negative electron affinities than their neighbors in groups 3A and 5A (see Figure 7.11). Suggest an explanation. 1A 8A He 73 2A 3A 4A 5A 6A 7A >0 Li Be -27 -122...
-
A hydrogen atom contains one electron and one proton. (a) Find the electric force between the proton and the electron. (The distance between an electron and a proton in a hydrogen atom is 5.29 x 1011...
-
The Classical Hydrogen Atom The electron in a hydrogen atom can be considered to be in a circular orbit with a radius of 0.0529 nm and a kinetic energy of 13.6 eV. If the electron behaved...
-
A score of X = 75 is measured in a population with a mean of = 100. A z-score of z = +1.50 is calculated. Without knowing the standard deviation, explain why the z-score of z = +1.50 is incorrect.
-
Alpha Corporation has 25 million shares of common stock issued and outstanding. On August 31 the board of directors voted a $1.20 per share cash dividend to stockholders of record as of September 5,...
-
In Exercises 109111, simplify each rational expression. Also, list all numbers that must be excluded from the domain. x + 2x x + 4x + 4 2
-
A rock climber accidentally drops a \(4.5-\mathrm{kg}\) backpack, and it falls \(160 \mathrm{~m}\) to the ground below. What is the change in the gravitational potential energy of the system...
-
In Rooney Company, direct labor is $20 per hour. The company expects to operate at 10,000 direct labor hours each month. In January 2017, direct labor totaling $206,000 is incurred in working 10,400...
-
One key limitation of Sage 50 Accounting software, while there is a Sage 50 cloud version that offers some cloud-based features, the mobile access and functionality may not be as extensive as with...
-
A double pipe heat exchanger is made of a 6-nom sch 40 commercial steel outer pipe and a 5-nom sch 40S stainless steel inner pipe. The fluid in the annular space is cyclohexane that has a volumetric...
-
1. Develop a clear profile of your typical customer by describing them in relevant demographic terms including: age, gender, income, marital status, education, religion, profession, and geographic...
-
C. Read the article. Then, identify the claim on the text and think about your assertion and counterclaim related to the text read. Write your answers on the table that follows, MANILA, Philippines...
-
What is the result of each of the following statements? [In the event a list is an answer, just write the list not the annotated version - Example: (a b c) NOT (list a b c) a. (car' (a (b c d))) b....
-
Calculate the fraction of the original amplitude obtained when a 560 load is connected to the output via a coupling capacitor (Cout in Fig. 3) to ground. HINT: the 5602 resistor will be in series...
-
Write a Scheme/Lisp expression to pull the value pear out of the following list use only car and cdr, no other combinations. (orange (apple grape ((pear) raisin) ) lemon) 8. (2) Reduce the above...
-
The Summit Petroleum Corporation will purchase an asset that qualifies for three-year MACRS depreciation. The cost is $250,000 and the asset will provide the following stream of earnings before...
-
Assuming the below results were obtained in a study used to test the accuracy of the rapid diagnostic test for influenza, calculate and interpret the positive predictive value (PPV) of the rapid...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
Classify each reaction as an oxidation, a reduction, or nether. (a) CH3 - CH2OH (b) (c) (d) (e) (f) g) (h) (i) (j) (k) (l) CrOs pyridine CH H2CrO4 CH3CH -- CH3 H3C CH3 CH3---CH3 LiAIH TiCI...
-
Predict the product(s) you would expect from treatment of each compound with (1) dilute, neutral KMnO4 and (2) warm, basic KMnO4 then dilute acid. (a) hex-1-yne (b) hex-2-yne (c) hex-3-yne (d)...
-
Cellosolve is the trade name for 2-ethoxyethanol, a common industrial solvent. This compound is produced in chemical plants that use ethylene as their only organic feedstock. Show how you would...
-
Action Quest Games adjusts its accounts annually. The following information is available for the year ended December 31, 2025. 1. Purchased a 1-year insurance policy on June 1 for $1,800 cash. 2....
-
With each pass of a comet about the Sun, the comets mass (a) remains virtually unchanged. (b) actually increases. (c) is appreciably reduced.
-
Why is carbon such a special atom?

Study smarter with the SolutionInn App



