Question: The Lewis structure for methylamine, CH3NH2, is shown below. Each CH bond is formed by the overlap of a(n) orbital from C and a(n) orbital
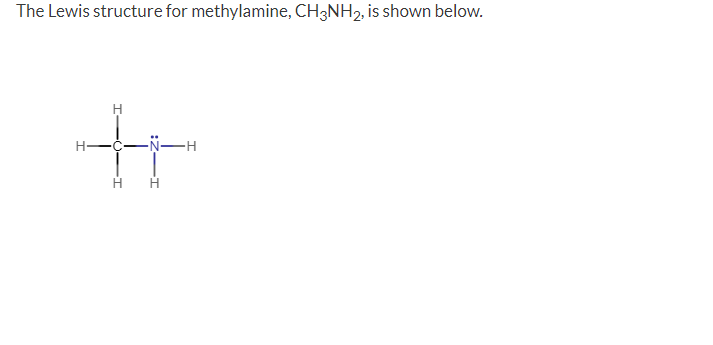
The Lewis structure for methylamine, CH3NH2, is shown below. Each CH bond is formed by the overlap of a(n) orbital from C and a(n) orbital from H. Part 2 (0.5 point) bond and is formed by the overlap of a(n) orbital from C and a(n) The CN bond is a orbital from N. Part 3 (0.2 point) orbital. The N lone pair is located in a(n)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


