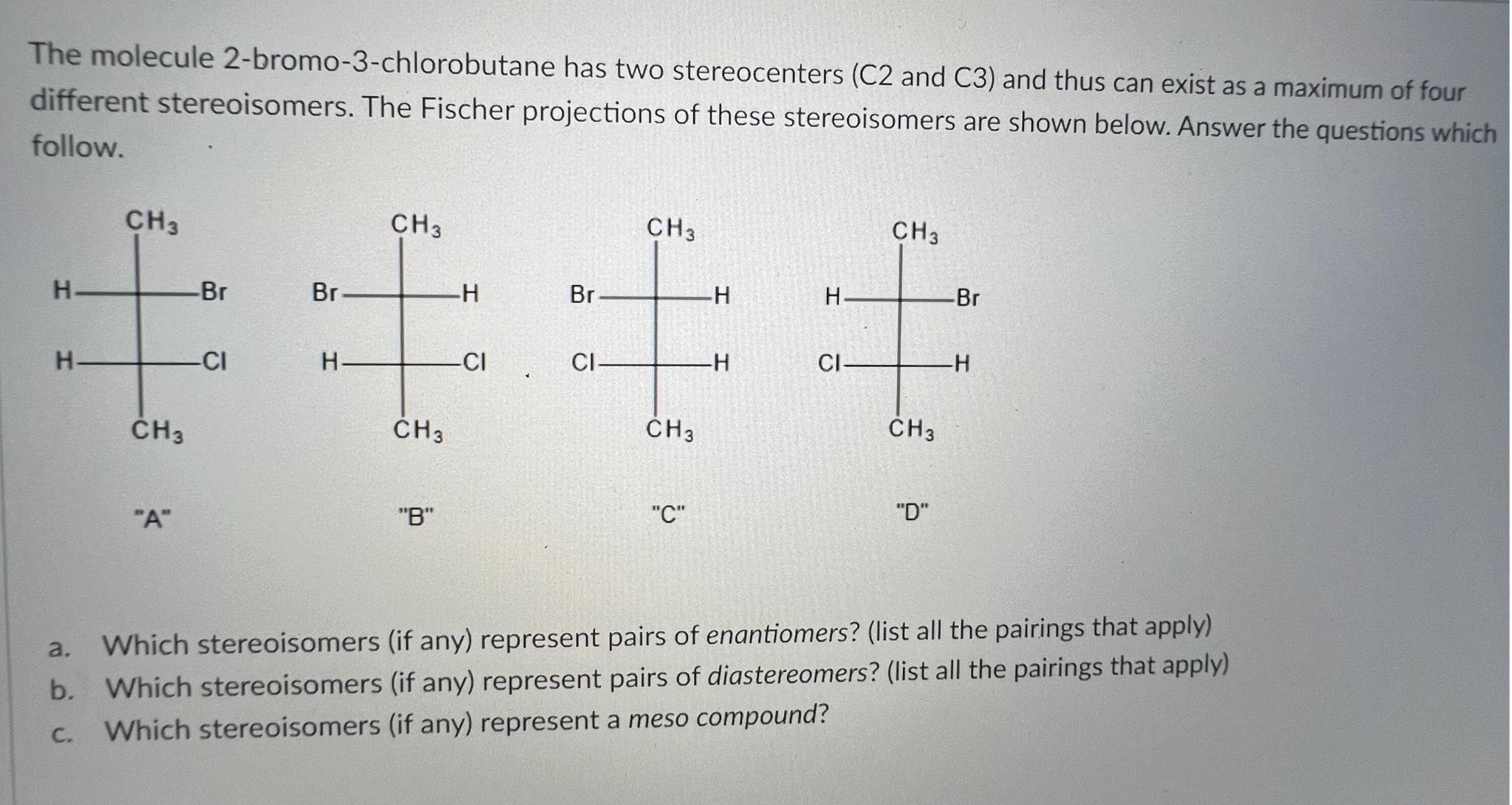
Question: The molecule 2 - bromo - 3 - chlorobutane has two stereocenters ( C 2 and C 3 ) and thus can exist as a
The molecule bromochlorobutane has two stereocenters and and thus can exist as a maximum of four different stereoisomers. The Fischer projections of these stereoisomers are shown below. Answer the questions which follow.
B
C
D
a Which stereoisomers if any represent pairs of enantiomers? list all the pairings that apply
b Which stereoisomers if any represent pairs of diastereomers? list all the pairings that apply
c Which stereoisomers if any represent a meso compound?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


