Question: The nucleic acid thymine when drawn as follows does not appear to be aromatic. Draw a resonance structure that shows the ring with aromatic character
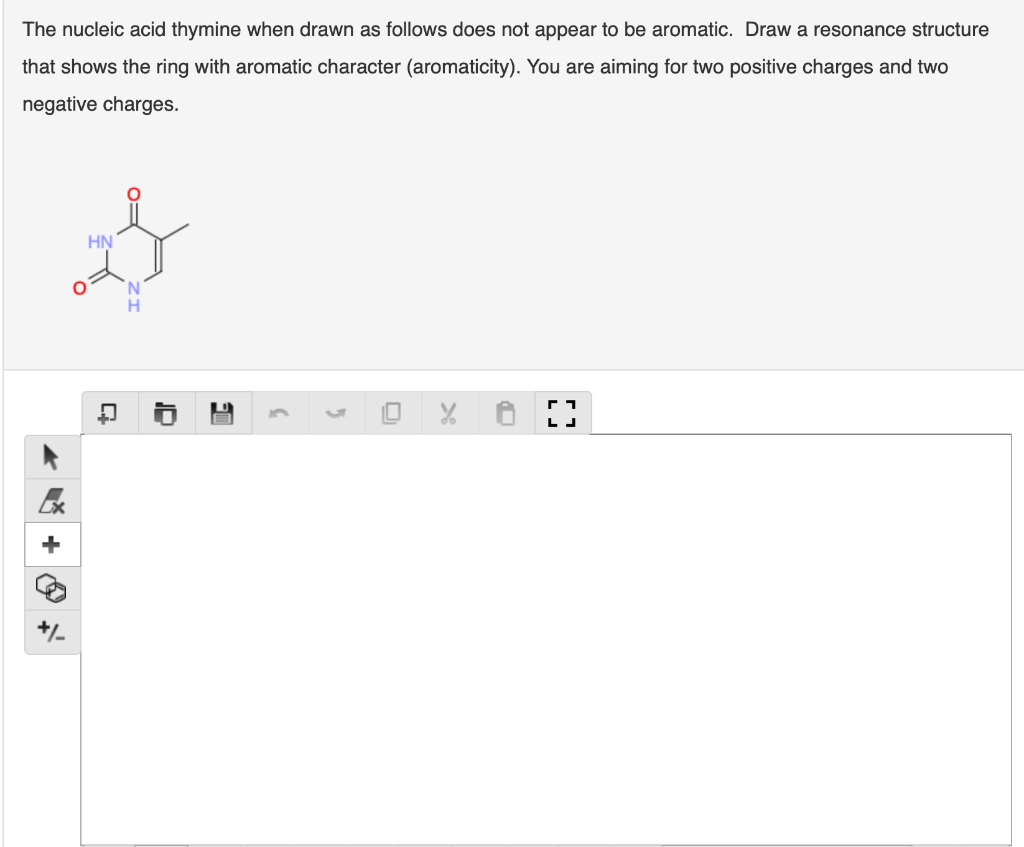
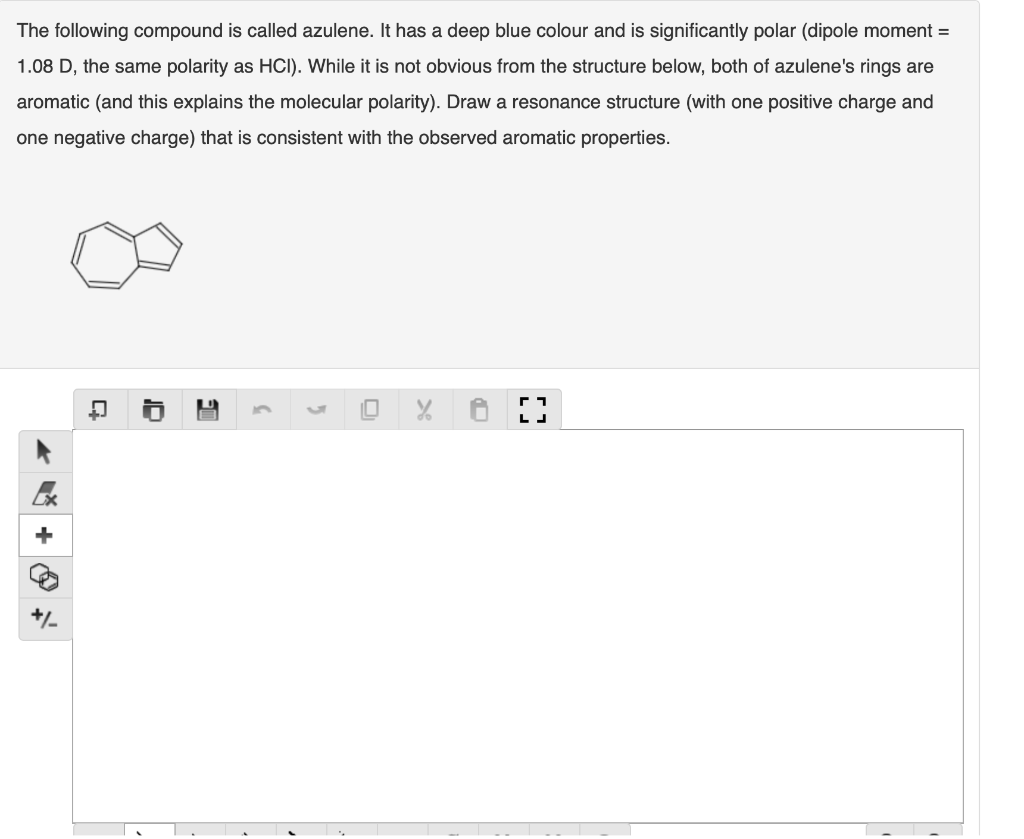
The nucleic acid thymine when drawn as follows does not appear to be aromatic. Draw a resonance structure that shows the ring with aromatic character (aromaticity). You are aiming for two positive charges and two negative charges. HN A o I 3 5 r LJ * + The following compound is called azulene. It has a deep blue colour and is significantly polar (dipole moment = 1.08 D, the same polarity as HCI). While it is not obvious from the structure below, both of azulene's rings are aromatic (and this explains the molecular polarity). Draw a resonance structure (with one positive charge and one negative charge) that is consistent with the observed aromatic properties. . C LJ Ex + +/
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


