Question: The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic
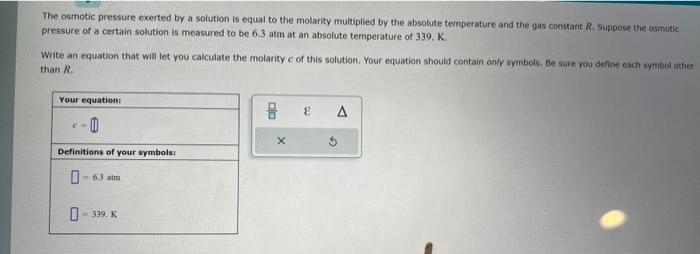
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 6.3 atm at an absolute temperature of 339.K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols, Be sure you deline each wymbol other than M. \begin{tabular}{|l|} \hline Your equationi \\ \hlinee= II \\ \hline Definitions of your symbols: \\ \hline \\ - 6.3 aim \\ = 339. K \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


