Question: the page was split they are the same question. I also need to report the extent of the reaction and the fractional conversion of each

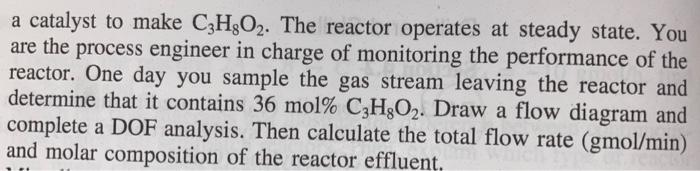
1,3-propanediol (C3H8O2) is a building block in the synthesis of polymers that are used to make fabrics or plastic bottles. 1,3-propanediol is made commercially by both chemical and biological routes. One chemical route is called hydroformylation, starting from ethylene oxide (C2H4O) C2H4O+CO+2H2C3H8O2 A gas stream containing 30mol%C2H4Or30mol%CO, and 40mol% H2 is fed to a reactor at 900gmol/min, where the mixture reacts over a catalyst to make C3H8O2. The reactor operates at steady state. You are the process engineer in charge of monitoring the performance of the reactor. One day you sample the gas stream leaving the reactor and determine that it contains 36mol%C3H8O2. Draw a flow diagram and complete a DOF analysis. Then calculate the total flow rate (gmol/min) and molar composition of the reactor effluent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


