Question: The parallel first-order reactions: A B Reaction 1 A Reaction 2 = have activation energies of Ej = 60,000 J/mol and E2 = 50,000 J/mol.
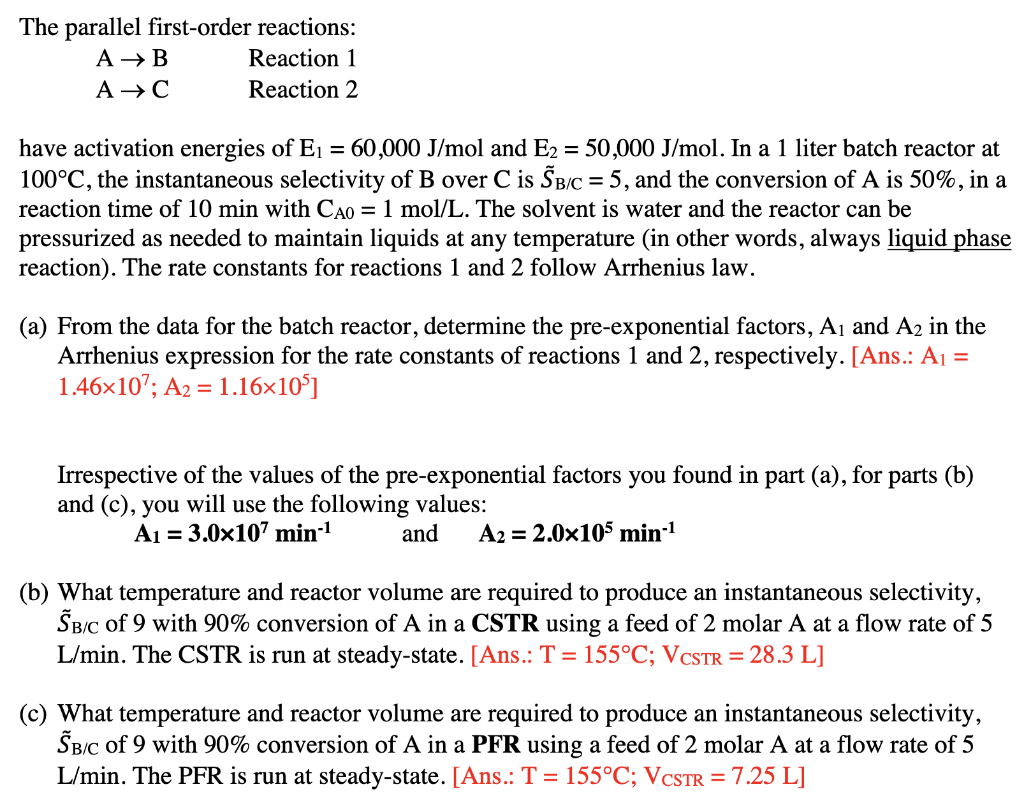
The parallel first-order reactions: A B Reaction 1 A Reaction 2 = have activation energies of Ej = 60,000 J/mol and E2 = 50,000 J/mol. In a 1 liter batch reactor at 100C, the instantaneous selectivity of B over C is B/C = 5, and the conversion of A is 50%, in a reaction time of 10 min with Cao = 1 mol/L. The solvent is water and the reactor can be pressurized as needed to maintain liquids at any temperature (in other words, always liquid phase reaction). The rate constants for reactions 1 and 2 follow Arrhenius law. (a) From the data for the batch reactor, determine the pre-exponential factors, Aj and A2 in the Arrhenius expression for the rate constants of reactions 1 and 2, respectively. [Ans.: Aj = 1.46x10; A2 = 1.16x10>] = Irrespective of the values of the pre-exponential factors you found in part (a), for parts (b) and (c), you will use the following values: A1 = 3.0x107 min-1 and A2 = 2.0x105 min-1 (b) What temperature and reactor volume are required to produce an instantaneous selectivity, Sbic of 9 with 90% conversion of A in a CSTR using a feed of 2 molar A at a flow rate of 5 L/min. The CSTR is run at steady-state. [Ans.: T = 155C; VCSTR = 28.3 L] (c) What temperature and reactor volume are required to produce an instantaneous selectivity, Bic of 9 with 90% conversion of A in a PFR using a feed of 2 molar A at a flow rate of 5 L/min. The PFR is run at steady-state. [Ans.: T = 155C; VCSTR = 7.25 L]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


