Question: The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in
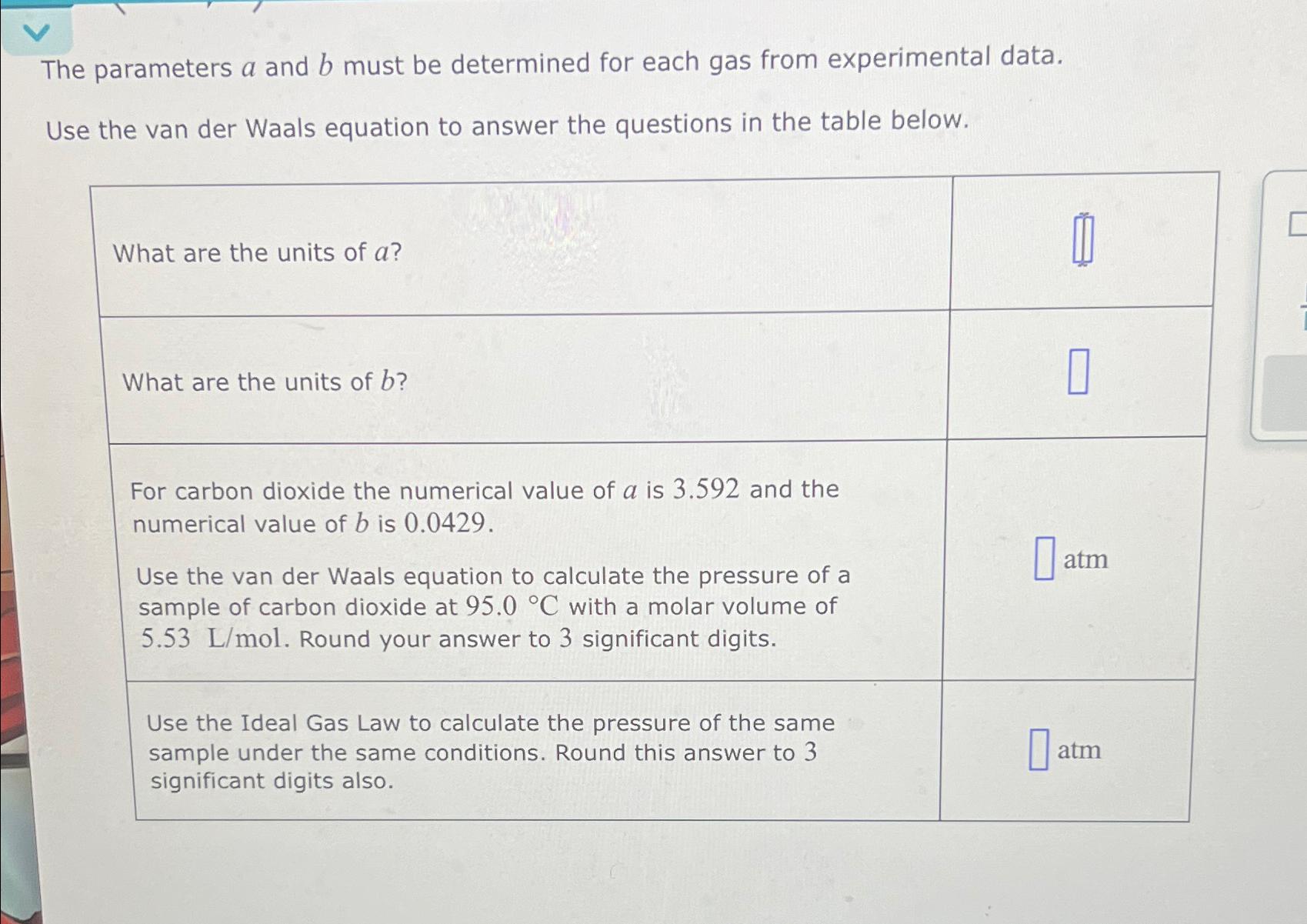
The parameters a and must be determined for each gas from experimental data.
Use the van der Waals equation to answer the questions in the table below.
What are the units of
What are the units of
For carbon dioxide the numerical value of is and the numerical value of is
Use the van der Waals equation to calculate the pressure of a sample of carbon dioxide at with a molar volume of Lmol Round your answer to significant digits.
Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round this answer to significant digits also.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


