Question: The percent ionic character of a bond between elements A and B may be approximated by the expression: where x_(A) and x_(B) are the
The percent ionic character of a bond between elements
Aand
Bmay be approximated by the expression:\ \ where
x_(A)and
x_(B)are the electronegativities for the respective elements. Compute the percents ionic character of the interatomic bonds for the following compounds: A)
TiO_(2), C)
KCl,D, E)
Femetal, and
F)
Fe_(2)O_(3).
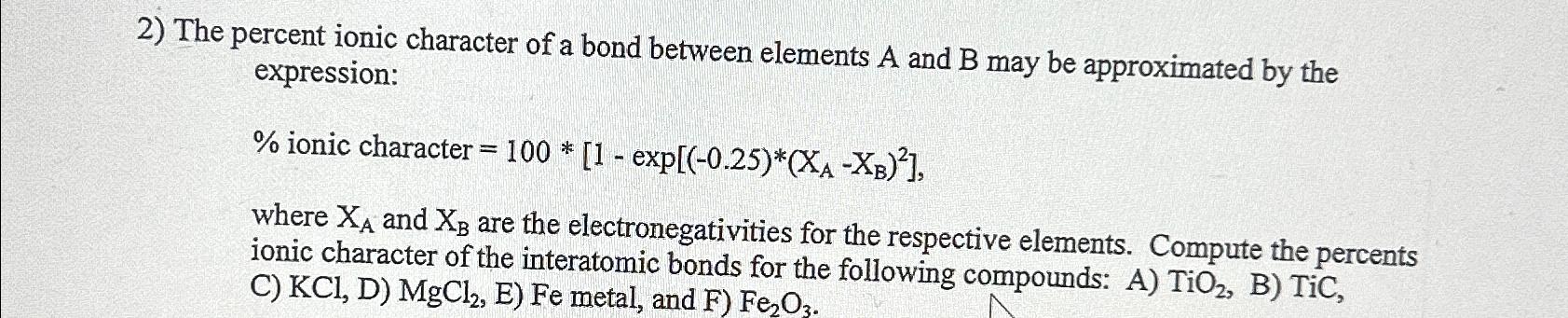
2) The percent ionic character of a bond between elements A and B may be approximated by the expression: %ioniccharacter=100[1exp[(0.25)(XAXB)2], where XA and XB are the electronegativities for the respective elements. Compute the percents ionic character of the interatomic bonds for the following compounds: A) TiO2, B) TiC, C) KCl,D)MgCl2,E)Fe metal, and F)Fe2O3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


