Question: The pH of a solution can be determined using the formula PH = -log10(C) where C is the concentration of hydrogen ions in the solution.
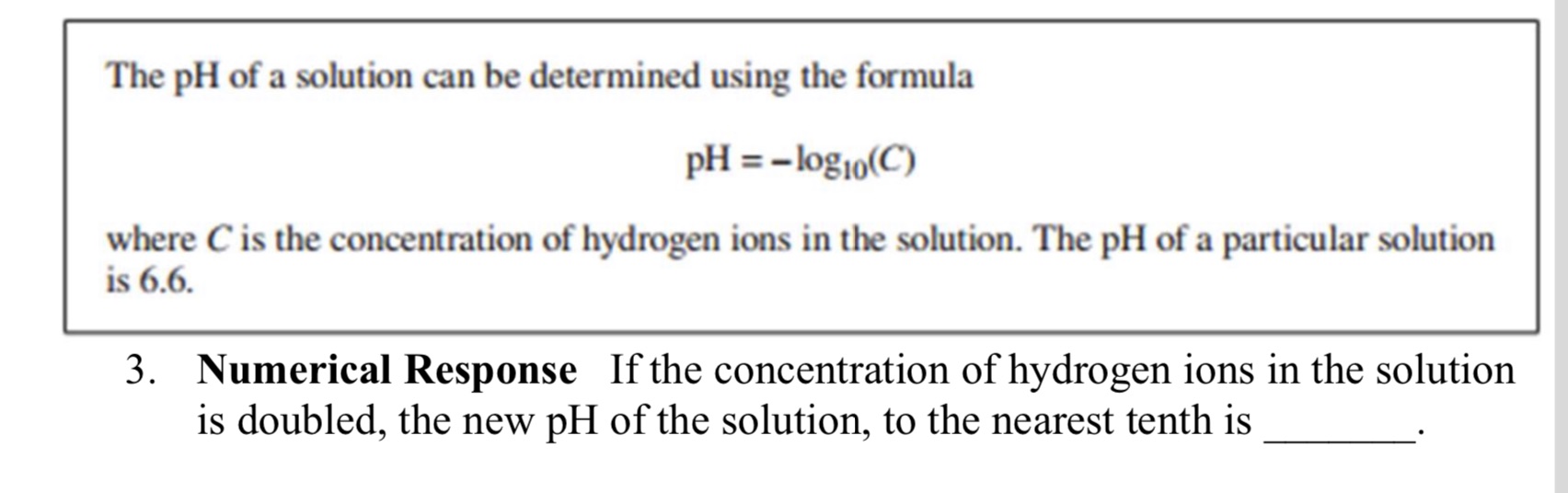
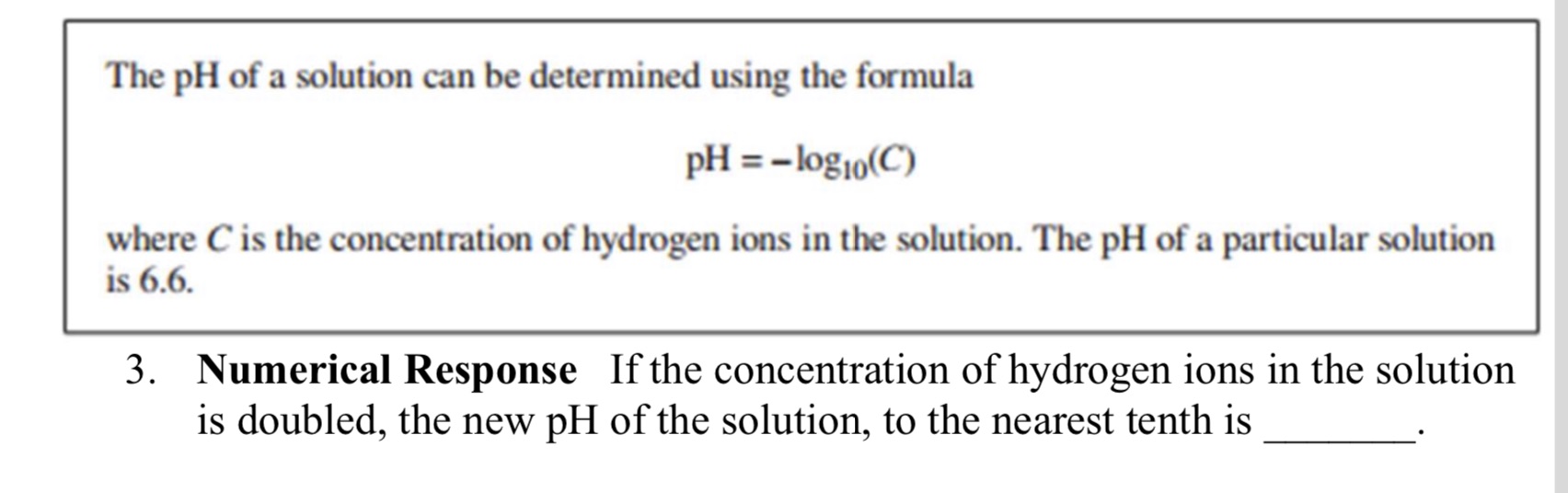
The pH of a solution can be determined using the formula PH = -log10(C) where C is the concentration of hydrogen ions in the solution. The pH of a particular solution is 6.6. 3. Numerical Response If the concentration of hydrogen ions in the solution is doubled, the new pH of the solution, to the nearest tenth is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


